Cell-Derived Plasma Membrane Vesicles Are Permeable to Hydrophilic Macromolecules
- PMID: 32053777
- PMCID: PMC7091462
- DOI: 10.1016/j.bpj.2019.12.040
Cell-Derived Plasma Membrane Vesicles Are Permeable to Hydrophilic Macromolecules
Abstract
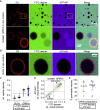
Giant plasma membrane vesicles (GPMVs) are a widely used experimental platform for biochemical and biophysical analysis of isolated mammalian plasma membranes (PMs). A core advantage of these vesicles is that they maintain the native lipid and protein diversity of the PM while affording the experimental flexibility of synthetic giant vesicles. In addition to fundamental investigations of PM structure and composition, GPMVs have been used to evaluate the binding of proteins and small molecules to cell-derived membranes and the permeation of drug-like molecules through them. An important assumption of such experiments is that GPMVs are sealed, i.e., that permeation occurs by diffusion through the hydrophobic core rather than through hydrophilic pores. Here, we demonstrate that this assumption is often incorrect. We find that most GPMVs isolated using standard preparations are passively permeable to various hydrophilic solutes as large as 40 kDa, in contrast to synthetic giant unilamellar vesicles. We attribute this leakiness to stable, relatively large, and heterogeneous pores formed by rupture of vesicles from cells. Finally, we identify preparation conditions that minimize poration and allow evaluation of sealed GPMVs. These unexpected observations of GPMV poration are important for interpreting experiments utilizing GPMVs as PM models, particularly for drug permeation and membrane asymmetry.
Copyright © 2020 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Sezgin E., Kaiser H.J., Levental I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012;7:1042–1051. - PubMed
-
- Levental K.R., Levental I. Giant plasma membrane vesicles: models for understanding membrane organization. Curr. Top. Membr. 2015;75:25–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

