Distinct Roles of mTOR Targets S6K1 and S6K2 in Breast Cancer
- PMID: 32054043
- PMCID: PMC7072743
- DOI: 10.3390/ijms21041199
Distinct Roles of mTOR Targets S6K1 and S6K2 in Breast Cancer
Abstract
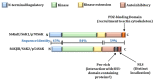
The mechanistic target of rapamycin (mTOR) is a master regulator of protein translation, metabolism, cell growth and proliferation. It forms two complexes, mTOR complex 1 (mTORC1) and 2 (mTORC2). mTORC1 is frequently deregulated in many cancers, including breast cancer, and is an important target for cancer therapy. The immunosuppressant drug rapamycin and its analogs that inhibit mTOR are currently being evaluated for their potential as anti-cancer agents, albeit with limited efficacy. mTORC1 mediates its function via its downstream targets 40S ribosomal S6 kinases (S6K) and eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1). There are two homologs of S6K: S6K1 and S6K2. Most of the earlier studies focused on S6K1 rather than S6K2. Because of their high degree of structural homology, it was generally believed that they behave similarly. Recent studies suggest that while they may share some functions, they may also exhibit distinct or even opposite functions. Both homologs have been implicated in breast cancer, although how they contribute to breast cancer may differ. The purpose of this review article is to compare and contrast the expression, structure, regulation and function of these two S6K homologs in breast cancer.
Keywords: RPS6KB1; RPS6KB2; S6K1; S6K2; breast cancer; mTOR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

