Activation of Astroglial Connexin is Involved in Concentration-Dependent Double-Edged Sword Clinical Action of Clozapine
- PMID: 32054069
- PMCID: PMC7072131
- DOI: 10.3390/cells9020414
Activation of Astroglial Connexin is Involved in Concentration-Dependent Double-Edged Sword Clinical Action of Clozapine
Abstract
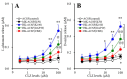
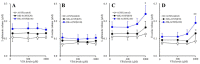
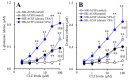
Clozapine (CLZ) is a gold-standard antipsychotic against treatment-refractory schizophrenia, but is one of the most toxic antipsychotic agents. Pharmacological mechanisms of the double-edged sword clinical action of CLZ remain to be clarified. To explore the mechanisms of CLZ, the present study determined the astroglial transmission associated with connexin43 (Cx43), which is the most principal expression in astrocytes and myocardial cells, and expression of Cx43 in primary cultured astrocytes. Both acute and subchronic administrations of CLZ concentration-dependently increased Cx43-associated astroglial release of l-glutamate and d-serine, whereas therapeutic-relevant concentration of CLZ acutely did not affect but subchronically increased astroglial release. In contrast, after the subchronic administration of therapeutic-relevant concentration of valproate (VPA), acute administration of therapeutic-relevant concentration of CLZ drastically increased Cx43-associated astroglial releases. VPA increased Cx43 expression in cytosol fraction without affecting plasma membrane fraction, whereas CLZ increased Cx43 expression in both fractions. Acute administration of therapeutic-relevant concentration of CLZ drastically increased Cx43 expression in the plasma membrane fraction of astrocytes subchronically treated with VPA. The present findings suggest that CLZ-induced the activation of Cx43-associated channel activity and transported Cx43 to plasma membrane, probably contribute to the double-edged sword clinical action of CLZ, such as improvement of cognitive dysfunction and CLZ-induced myocarditis.
Keywords: clozapine; connexin; hemichannel; schizophrenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lieberman J.A., Bymaster F.P., Meltzer H.Y., Deutch A.Y., Duncan G.E., Marx C.E., Aprille J.R., Dwyer D.S., Li X.M., Mahadik S.P., et al. Antipsychotic drugs: Comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol. Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. - DOI - PMC - PubMed
-
- Meltzer H.Y., Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog. Brain Res. 2008;172:177–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

