Coordinated alteration of mRNA-microRNA transcriptomes associated with exosomes and fatty acid metabolism in adipose tissue and skeletal muscle in grazing cattle
- PMID: 32054170
- PMCID: PMC7649083
- DOI: 10.5713/ajas.19.0682
Coordinated alteration of mRNA-microRNA transcriptomes associated with exosomes and fatty acid metabolism in adipose tissue and skeletal muscle in grazing cattle
Abstract
Objective: On the hypothesis that grazing of cattle prompts organs to secrete or internalize circulating microRNAs (c-miRNAs) in parallel with changes in energy metabolism, we aimed to clarify biological events in adipose, skeletal muscle, and liver tissues in grazing Japanese Shorthorn (JSH) steers by a transcriptomic approach.
Methods: The subcutaneous fat (SCF), biceps femoris muscle (BFM), and liver in JSH steers after three months of grazing or housing were analyzed using microarray and quantitative polymerase chain reaction (qPCR), followed by gene ontology (GO) and functional annotation analyses.
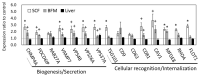
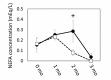
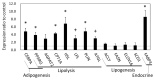
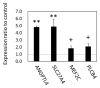
Results: The results of transcriptomics indicated that SCF was highly responsive to grazing compared to BFM and liver tissues. The 'Exosome', 'Carbohydrate metabolism' and 'Lipid metabolism' were extracted as the relevant GO terms in SCF and BFM, and/or liver from the >1.5-fold-altered mRNAs in grazing steers. The qPCR analyses showed a trend of upregulated gene expression related to exosome secretion and internalization (charged multivesicular body protein 4A, vacuolar protein sorting-associated protein 4B, vesicle associated membrane protein 7, caveolin 1) in the BFM and SCF, as well as upregulation of lipolysisassociated mRNAs (carnitine palmitoyltransferase 1A, hormone-sensitive lipase, perilipin 1, adipose triglyceride lipase, fatty acid binding protein 4) and most of the microRNAs (miRNAs) in SCF. Moreover, gene expression related to fatty acid uptake and inter-organ signaling (solute carrier family 27 member 4 and angiopoietin-like 4) was upregulated in BFM, suggesting activation of SCF-BFM organ crosstalk for energy metabolism. Meanwhile, expression of plasma exosomal miR-16a, miR-19b, miR-21-5p, and miR-142-5p was reduced. According to bioinformatic analyses, the c-miRNA target genes are associated with the terms 'Endosome', 'Caveola', 'Endocytosis', 'Carbohydrate metabolism', and with pathways related to environmental information processing and the endocrine system.
Conclusion: Exosome and fatty acid metabolism-related gene expression was altered in SCF of grazing cattle, which could be regulated by miRNA such as miR-142-5p. These changes occurred coordinately in both the SCF and BFM, suggesting involvement of exosome in the SCF-BFM organ crosstalk to modulate energy metabolism.
Keywords: Exosome; Grazing Cattle; Lipid Metabolism; Organ Crosstalk; Subcutaneous Fat; microRNA.
Conflict of interest statement
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Figures



Similar articles
-
Differences in Circulating microRNAs between Grazing and Grain-Fed Wagyu Cattle Are Associated with Altered Expression of Intramuscular microRNA, the Potential Target PTEN, and Lipogenic Genes.PLoS One. 2016 Sep 9;11(9):e0162496. doi: 10.1371/journal.pone.0162496. eCollection 2016. PLoS One. 2016. PMID: 27611783 Free PMC article.
-
Grazing Affects Exosomal Circulating MicroRNAs in Cattle.PLoS One. 2015 Aug 26;10(8):e0136475. doi: 10.1371/journal.pone.0136475. eCollection 2015. PLoS One. 2015. PMID: 26308447 Free PMC article.
-
Grazing-induced changes in muscle microRNA-206 and -208b expression in association with myogenic gene expression in cattle.Anim Sci J. 2015 Nov;86(11):952-60. doi: 10.1111/asj.12381. Epub 2015 Jun 30. Anim Sci J. 2015. PMID: 26122272
-
Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte.Metabolism. 2020 Feb;103:154006. doi: 10.1016/j.metabol.2019.154006. Epub 2019 Nov 10. Metabolism. 2020. PMID: 31715176
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
Cited by
-
Variation of miRNA Content in Cow Raw Milk Depending on the Dairy Production System.Int J Mol Sci. 2022 Oct 2;23(19):11681. doi: 10.3390/ijms231911681. Int J Mol Sci. 2022. PMID: 36232984 Free PMC article.
-
Maternal Nutrient Restriction Disrupts Gene Expression and Metabolites Associated with Urea Cycle, Steroid Synthesis, Glucose Homeostasis, and Glucuronidation in Fetal Calf Liver.Metabolites. 2022 Feb 24;12(3):203. doi: 10.3390/metabo12030203. Metabolites. 2022. PMID: 35323646 Free PMC article.
-
Composition, isolation, identification and function of adipose tissue-derived exosomes.Adipocyte. 2021 Dec;10(1):587-604. doi: 10.1080/21623945.2021.1983242. Adipocyte. 2021. PMID: 34709975 Free PMC article. Review.
-
Comprehensive MicroRNA Expression Profile of the Mammary Gland in Lactating Dairy Cows With Extremely Different Milk Protein and Fat Percentages.Front Genet. 2020 Dec 3;11:548268. doi: 10.3389/fgene.2020.548268. eCollection 2020. Front Genet. 2020. PMID: 33343617 Free PMC article.
-
Extracellular vesicle-derived miRNA-mediated cell-cell communication inference for single-cell transcriptomic data with miRTalk.Genome Biol. 2025 Apr 14;26(1):95. doi: 10.1186/s13059-025-03566-x. Genome Biol. 2025. PMID: 40229908 Free PMC article.
References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

