A functional antibody cross-reactive to both human and murine cytotoxic T-lymphocyte-associated protein 4 via binding to an N-glycosylation epitope
- PMID: 32054416
- PMCID: PMC7039627
- DOI: 10.1080/19420862.2020.1725365
A functional antibody cross-reactive to both human and murine cytotoxic T-lymphocyte-associated protein 4 via binding to an N-glycosylation epitope
Abstract
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152) is a receptor on T cells that inhibits the cell's functions. Blocking CTLA-4 with an antibody has proven effective for the treatment of cancer patients. Anti-CTLA-4 antibodies currently approved for clinical use can bind to human CTLA-4, but do not cross-react to murine CTLA-4. Here, we report the generation and characterization of a functional humanized antibody, mAb146, against both human and murine CTLA-4. Alanine scanning of CTLA-4 using mammalian cell expression cassette identified the unique epitopes of this novel antibody. In addition to the amino acid residues interacting with ligands CD80 and CD86, an N-glycosylation site on N110, conserved in CTLA-4 of human, monkey, and mouse, was identified as the specific epitope that might contribute to the cross-species binding and function of this antibody. This finding may also contribute to the understanding of the glycosylation of CTLA-4 and its related biologic function. In addition to facilitating preclinical development of anti-CTLA-4 antibodies, mAb146 may be useful as a therapeutic agent.
Keywords: CD152; Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4); N-glycosylation; antibody; cross-species binding; epitope mapping.
Figures



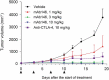





References
-
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi:10.1084/jem.20130579. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
