Adverse event rates and economic burden associated with purine nucleoside analogs in patients with hairy cell leukemia: a US population-retrospective claims analysis
- PMID: 32054500
- PMCID: PMC7020358
- DOI: 10.1186/s13023-020-1325-9
Adverse event rates and economic burden associated with purine nucleoside analogs in patients with hairy cell leukemia: a US population-retrospective claims analysis
Abstract
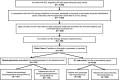
Background: Purine nucleoside analogs (PNAs) are the recommended first-line treatment for patients with hairy cell leukemia (HCL), but they are associated with adverse events (AEs). Due to a lack of real-world evidence regarding AEs that are associated with PNAs, we used commercial data to assess AE rates, AE-related health care resource utilization (HCRU), and costs among PNA-treated patients with HCL. Adults aged ≥18 years with ≥2 claims for HCL ≥30 days apart from 1 January 2006 through 31 December 2015 were included. Included patients had ≥1 claim for HCL therapy (cladribine ± rituximab or pentostatin ± rituximab [index date: first claim date]) and continuous enrollment for a ≥ 6-month baseline and ≥ 12-month follow-up period. Patient sub-cohorts were based on the occurrence of myelosuppression and opportunistic infections (OIs). Generalized linear models were used to compare HCRU and costs.
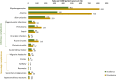
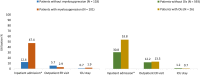
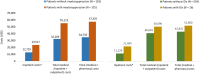
Results: In total, 647 PNA-treated patients were identified (mean age: 57.1 years). Myelosuppression and OI incidence were 461 and 42 per 1000 patient-years, respectively. Adjusted results indicated that those with myelosuppression had higher rates of hospitalization (47.4% vs 12.4%; P < .0001) and incurred higher mean inpatient costs ($23,517 vs $12,729; P = .011) and total costs ($57,325 vs $34,733; P = .001) as compared with those without myelosuppression. Similarly, patients with OIs had higher rates of hospitalization (53.8% vs 30.8%; P = .025) and incurred higher mean inpatient costs ($21,494 vs $11,229; P < .0001) as compared with those without OIs.
Conclusions: PNA therapy is highly effective but associated with significant toxicities that increase costs; these findings indicate a need for therapies with improved toxicity profiles and better risk stratification of patients at risk of developing myelosuppression and OIs.
Keywords: Adverse events; Hairy cell leukemia; Myelosuppression; Purine nucleoside analogs.
Conflict of interest statement
N Epperla, H Yuce, and L Andritsos have no conflicts to disclose.
M Pavilack, T Olufade, and S Kabadi are employees of AstraZeneca, the study sponsor.
R Bashyal and J Li are employees of STATinMED Research, a paid consultant to the study sponsor.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

