Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials
- PMID: 32054504
- PMCID: PMC7020579
- DOI: 10.1186/s13075-020-2120-7
Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials
Abstract
Background: Colchicine is a widely used drug to treat inflammatory diseases. Due to its long historical use in medicine, controlled clinical trials have been small and there remains some caution with the use of this drug in patients with co-morbidities. The aim of the study is to systematically examine the side effect profile of colchicine in controlled clinical trials across all published indications.
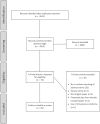
Methods: A systematic review was conducted in accordance with PRISMA methodology. The Cochrane Library, MEDLINE and EMBASE were searched for double-blind controlled trials of oral colchicine in adult patients that reported adverse event data. Meta-analyses were used to determine the relative risk (RR) of adverse events in colchicine users compared to comparator groups.
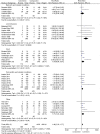
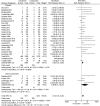
Results: A total of 4915 studies were initially identified and after exclusions, 35 randomised controlled trials with placebo (n = 35) or active comparators (n = 5) were included. The most common diseases studied were gout, liver cirrhosis and pericarditis. There were a total of 8659 pooled participants, 4225 participants were randomised to receive colchicine, 3956 to placebo and 411 to an active comparator. Diarrhoea was reported in 17.9% of colchicine users versus 13.1% in comparator groups (RR 2.4, 95% confidence interval (CI) 1.6, 3.7). Any gastrointestinal event was reported in 17.6% of colchicine users and 13.1% of comparators (RR 1.7, 95% CI 1.3, 2.3). Adverse liver events were reported in 1.9% of colchicine users versus 1.1% in the comparator groups (RR 1.6, 95% CI 0.9, 3.0). Muscle events were reported in 4.2% of colchicine users and 3.3% in the comparator groups (RR 1.3, 95% CI 0.8, 1.9). Haematology events were reported in 0.6% of colchicine users and 0.4% of comparator groups (RR 1.34 (0.64, 2.82). No study reported neuropathy events. Other sensory events were reported in 1.1% of colchicine users and 1.5% of comparator groups (RR 1.4, 95% CI 0.3, 6.7). Infectious events were reported in 0.4% of colchicine users and 2.1% of comparator groups (RR 1.0, 95% CI 0.7, 1.5). No study reported death as an adverse event.
Conclusion: Colchicine increases the rate of diarrhoea and gastrointestinal adverse events but does not increase the rate of liver, sensory, muscle, infectious or haematology adverse events or death.
Keywords: Colchicine; Diarrhoea; Gout; Nausea.
Conflict of interest statement
SS, KY, KA and PR declare no competing interests. Dr. Dalbeth reports grants and personal fees from AstraZeneca, grants from Amgen, personal fees from Dyve, personal fees from Hengrui, personal fees from Horizon, personal fees from Kowa, personal fees from Abbvie, personal fees from Pfizer, personal fees from Janssen, outside the submitted work.
Figures



References
-
- Evans TI, et al. A comprehensive investigation of inpatient intravenous colchicine use shows more education is needed. J Rheumatol. 1996;23(1):143–148. - PubMed
-
- Liberati A., Altman D. G, Tetzlaff J., Mulrow C., Gotzsche P. C, Ioannidis J. P A, Clarke M., Devereaux P J, Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700–b2700. doi: 10.1136/bmj.b2700. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

