Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study
- PMID: 32054512
- PMCID: PMC7020576
- DOI: 10.1186/s13287-020-1583-4
Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study
Abstract
Introduction: Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide. COPD results from chronic inflammation of the lungs. Current treatments, including physical and chemical therapies, provide limited results. Stem cells, particularly mesenchymal stem cells (MSCs), are used to treat COPD. Here, we evaluated the safety and efficacy of umbilical cord-derived (UC)-MSCs for treating COPD.
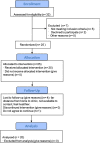
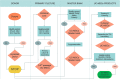
Methods: Twenty patients were enrolled, 9 at stage C and 11 at stage D per the Global Initiative for Obstructive Lung Disease (GOLD) classification. Patients were infused with 106 cells/kg of expanded allogeneic UC-MSCs. All patients were followed for 6 months after the first infusion. The treatment end-point included a comprehensive safety evaluation, pulmonary function testing (PFT), and quality-of-life indicators including questionnaires, the 6-min walk test (6MWT), and systemic inflammation assessments. All patients completed the full infusion and 6-month follow-up.
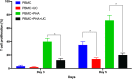
Results: No infusion-related toxicities, deaths, or severe adverse events occurred that were deemed related to UC-MSC administration. The UC-MSC-transplanted patients showed a significantly reduced Modified Medical Research Council score, COPD assessment test, and number of exacerbations. However, the forced expiratory volume in 1 s, C-reactive protein, and 6MWT values were nonsignificantly reduced after treatment (1, 3, and 6 months) compared with those before the treatment.
Conclusion: Systemic UC-MSC administration appears to be safe in patients with moderate-to-severe COPD, can significantly improve their quality of life, and provides a basis for subsequent cell therapy investigations.
Trial registration: ISRCTN, ISRCTN70443938. Registered 06 July 2019.
Keywords: COPD; Chronic obstructive pulmonary disease; Mesenchymal stem cells; Umbilical cord-derived mesenchymal stem cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Comment in
-
Comment on "Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study".Stem Cell Res Ther. 2020 Aug 5;11(1):340. doi: 10.1186/s13287-020-01859-5. Stem Cell Res Ther. 2020. PMID: 32758293 Free PMC article.
References
-
- Chang Y-P, Lai C-H, Lin C-Y, Chang Y-C, Lin M-C, Chong I-W, Sheu C-C, Wei Y-F, Chu K-A, Tsai J-R. Mortality and vertebral fracture risk associated with long-term oral steroid use in patients with chronic obstructive pulmonary disease: a systemic review and meta-analysis. Chronic Respir Dis. 2019;16:1479973119838280. doi: 10.1177/1479973119838280. - DOI - PMC - PubMed
-
- Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, Horn B, Yu L, Talano J-A, Nemecek E. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20(2):229–235. doi: 10.1016/j.bbmt.2013.11.001. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

