Multipotency of mouse trophoblast stem cells
- PMID: 32054514
- PMCID: PMC7020558
- DOI: 10.1186/s13287-020-1567-4
Multipotency of mouse trophoblast stem cells
Abstract
Background: In a number of disease processes, the body is unable to repair injured tissue, promoting the need to develop strategies for tissue repair and regeneration, including the use of cellular therapeutics. Trophoblast stem cells (TSCs) are considered putative stem cells as they differentiate into other subtypes of trophoblast cells. To identify cells for future therapeutic strategies, we investigated whether TSCs have properties of stem/progenitor cells including self-renewal and the capacity to differentiate into parenchymal cells of fetal organs, in vitro and in vivo.
Methods: TSCs were isolated using anti-CD117 micro-beads, from embryonic day 18.5 placentas. In vitro, CD117+ TSCs were cultured, at a limiting dilution in growth medium for the development of multicellular clones and in specialized medium for differentiation into lung epithelial cells, cardiomyocytes, and retinal photoreceptor cells. CD117+ TSCs were also injected in utero into lung, heart, and the sub-retinal space of embryonic day 13.5 fetuses, and the organs were harvested for histological assessment after a natural delivery.
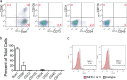
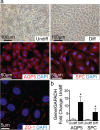
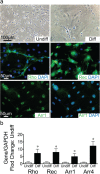
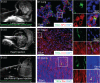
Results: We first identified CD117+ cells within the labyrinth zone and chorionic basal plate of murine placentas in late pregnancy, embryonic day 18.5. CD117+ TSCs formed multicellular clones that remained positive for CD117 in vitro, consistent with self-renewal properties. The clonal cells demonstrated multipotency, capable of differentiating into lung epithelial cells (endoderm), cardiomyocytes (mesoderm), and retinal photoreceptor cells (ectoderm). Finally, injection of CD117+ TSCs in utero into lungs, hearts, and the sub-retinal spaces of fetuses resulted in their engraftment on day 1 after birth, and the CD117+ TSCs differentiated into lung alveolar epithelial cells, heart cardiomyocytes, and retina photoreceptor cells, corresponding with the organs in which they were injected.
Conclusions: Our findings demonstrate that CD117+ TSCs have the properties of stem cells including clonogenicity, self-renewal, and multipotency. In utero administration of CD117+ TSCs engraft and differentiate into resident cells of the lung, heart, and retina during mouse development.
Keywords: In utero; Multipotency; Stem cells; Trophoblast cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

