Adipocyte-Induced FABP4 Expression in Ovarian Cancer Cells Promotes Metastasis and Mediates Carboplatin Resistance
- PMID: 32054768
- PMCID: PMC10656748
- DOI: 10.1158/0008-5472.CAN-19-1999
Adipocyte-Induced FABP4 Expression in Ovarian Cancer Cells Promotes Metastasis and Mediates Carboplatin Resistance
Abstract
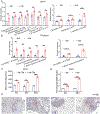
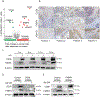
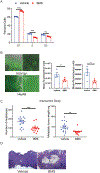
Adipocytes are critical for ovarian cancer cells to home to the omentum, but the metabolic changes initiated by this interaction are unknown. To this end, we carried out unbiased mass spectrometry-based metabolomic and proteomic profiling of cancer cells cocultured with primary human omental adipocytes. Cancer cells underwent significant proteo-metabolomic alteration(s), typified by changes in the lipidome with corresponding upregulation of lipid metabolism proteins. FABP4, a lipid chaperone protein, was identified as the critical regulator of lipid responses in ovarian cancer cells cocultured with adipocytes. Subsequently, knockdown of FABP4 resulted in increased 5-hydroxymethylcytosine levels in the DNA, downregulation of gene signatures associated with ovarian cancer metastasis, and reduced clonogenic cancer cell survival. In addition, clustered regularly interspaced short palindromic repeats (CRISPR)-mediated knockout of FABP4 in high-grade serous ovarian cancer cells reduced metastatic tumor burden in mice. Consequently, a small-molecule inhibitor of FABP4 (BMS309403) not only significantly reduced tumor burden in a syngeneic orthotopic mouse model but also increased the sensitivity of cancer cells toward carboplatin both in vitro and in vivo. Taken together, these results show that targeting FABP4 in ovarian cancer cells can inhibit their ability to adapt and colonize lipid-rich tumor microenvironments, providing an opportunity for specific metabolic targeting of ovarian cancer metastasis. SIGNIFICANCE: Ovarian cancer metastatic progression can be restricted by targeting a critical regulator of lipid responses, FABP4.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

