B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches
- PMID: 32055000
- PMCID: PMC7214244
- DOI: 10.1038/s41375-020-0734-z
B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches
Abstract
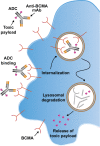
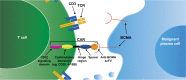
Despite considerable advances in the treatment of multiple myeloma (MM) in the last decade, a substantial proportion of patients do not respond to current therapies or have a short duration of response. Furthermore, these treatments can have notable morbidity and are not uniformly tolerated in all patients. As there is no cure for MM, patients eventually become resistant to therapies, leading to development of relapsed/refractory MM. Therefore, an unmet need exists for MM treatments with novel mechanisms of action that can provide durable responses, evade resistance to prior therapies, and/or are better tolerated. B-cell maturation antigen (BCMA) is preferentially expressed by mature B lymphocytes, and its overexpression and activation are associated with MM in preclinical models and humans, supporting its potential utility as a therapeutic target for MM. Moreover, the use of BCMA as a biomarker for MM is supported by its prognostic value, correlation with clinical status, and its ability to be used in traditionally difficult-to-monitor patient populations. Here, we review three common treatment modalities used to target BCMA in the treatment of MM: bispecific antibody constructs, antibody-drug conjugates, and chimeric antigen receptor (CAR)-modified T-cell therapy. We provide an overview of preliminary clinical data from trials using these therapies, including the BiTE® (bispecific T-cell engager) immuno-oncology therapy AMG 420, the antibody-drug conjugate GSK2857916, and several CAR T-cell therapeutic agents including bb2121, NIH CAR-BCMA, and LCAR-B38M. Notable antimyeloma activity and high minimal residual disease negativity rates have been observed with several of these treatments. These clinical data outline the potential for BCMA-targeted therapies to improve the treatment landscape for MM. Importantly, clinical results to date suggest that these therapies may hold promise for deep and durable responses and support further investigation in earlier lines of treatment, including newly diagnosed MM.
Conflict of interest statement
NS has received research funding from Celgene, Janssen, Bluebird Bio, and Sutro Biopharma; has served in an advisory role for Genentech, Seattle Genetics, Oncopeptides, Karyopharm, Surface Oncology, Precision BioSciences, GlaxoSmithKline, Nektar, Amgen, Indapta Therapeutics, and Sanofi; and owns stock in Indapta Therapeutics. AC has received research funding from Amgen, Celgene, Janssen, Millennium/Takeda, Novartis Pharmaceuticals, Pharmacyclics, and Seattle Genetics; has served in an advisory role for Amgen, Celgene, Janssen, Karyopharm, Novartis Pharmaceuticals, Oncopeptides, Sanofi, and Seattle Genetics; and has served as a consultant for Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium/Takeda, and Novartis Pharmaceuticals. ES and KM are employees and stockholders of Amgen. SZU has served as a consultant for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Sanofi, Seattle Genetics, and SkylineDx; has received speaker’s fees from Amgen, Celgene, Janssen, and Takeda; and has received research funding from Amgen, AbbVie, Array-Biopharma, Bristol-Myers Squibb, Celgene, Janssen, Pharmacyclics, Sanofi, Seattle Genetics, and SkylineDx.
Figures

References
-
- National Comprehensive Cancer Network. NCCN clinical Practice Guidelines in Oncology (NCCN Guidelines®). Multiple myeloma (version 2.2019). http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed 2 May 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

