Immunotherapeutic approaches for small-cell lung cancer
- PMID: 32055013
- PMCID: PMC7212527
- DOI: 10.1038/s41571-019-0316-z
Immunotherapeutic approaches for small-cell lung cancer
Abstract
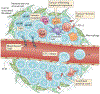
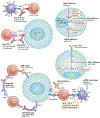
Immune-checkpoint inhibitors (ICIs) are approved in the first-line and third-line settings for patients with extensive-stage or relapsed small-cell lung cancer (SCLC), respectively. In the first-line setting, the addition of the anti-programmed cell death 1 ligand 1 (PD-L1) antibody atezolizumab to chemotherapy improves overall survival (OS). In patients with relapsed disease, data from nonrandomized trials have revealed promising responses, although a significant improvement in OS over that obtained with conventional chemotherapy was not achieved in a randomized trial in this setting. Substantial research interest exists in identifying predictive biomarkers that could guide the use of ICIs in patients with SCLC. PD-L1 expression is typically low or absent in SCLC, which has precluded its use as a predictive biomarker. Tumour mutational burden might have some predictive value, although blood-based measures of tumour mutational burden did not have predictive value in patients receiving atezolizumab plus chemotherapy in the first-line setting. After three decades, ICIs have finally enabled an improvement in OS for patients with SCLC; however, a substantial amount of research remains to be done, including identifying the optimal therapeutic strategy and predictive biomarkers. In this Review, we describe the available data on clinical efficacy, the emerging evidence regarding biomarkers and ongoing clinical trials using ICIs and other immunotherapies in patients with SCLC.
Conflict of interest statement
Competing interests
W.T.I. has acted as a consultant for Defined Health, Genentech and Outcomes Insights. L.H. reports clinical trial funding from BMS, Boehringer Ingelheim and Xcovery; and has acted as a consultant for AbbVie, AstraZeneca, EMD Serono, Incyte, Merck, Pfizer, Roche-Genentech, Tesaro and Xcovery. J.P declares no competing interests.
Figures
References
-
- Bernhardt EB & Jalal SI Small cell lung cancer. Cancer Treat. Res 170, 301–322 (2016). - PubMed
-
- Gazdar AF & Minna JD Developing new, rational therapies for recalcitrant small cell lung cancer. J. Natl Cancer Inst 108, djw119 (2016). - PubMed
-
- Horn L et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med 379, 2220–2229 (2018). - PubMed
-
- Antonia SJ et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 17, 883–895 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

