Reductive radical-polar crossover: traditional electrophiles in modern radical reactions
- PMID: 32055300
- PMCID: PMC7003961
- DOI: 10.1039/c9sc03359a
Reductive radical-polar crossover: traditional electrophiles in modern radical reactions
Abstract
The concept of reductive radical-polar crossover (RRPCO) reactions has recently emerged as a valuable and powerful tool to overcome limitations of both radical and traditional polar chemistry. Especially in case of additions to carbonyl compounds, the synergy of radical and polar pathways is of great advantage since it enables the use of traditional carbonyl electrophiles in radical reactions. The most recent and synthetically important transformations following this line are summarised in the first part of this review. The second part deals with transformations, in which the concept of RRPCO promotes the usage of alkyl halides as electrophiles in radical reactions.
This journal is © The Royal Society of Chemistry 2019.
Figures

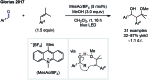
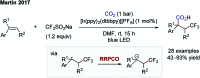
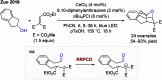


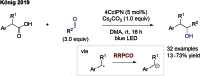

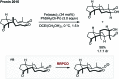
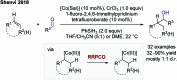

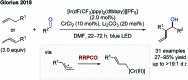


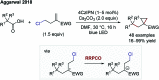



References
-
- Studer A., Curran D. P. Angew. Chem., Int. Ed. 2016;55:58–102. - PubMed
-
-
For selected examples, see:
- Wang C., Qin J., Shen X., Riedel R., Harms K., Meggers E. Angew. Chem., Int. Ed. 2016;55:685–688. - PubMed
- Li W., Duan Y., Zhang M., Cheng J., Zhu C. Chem. Commun. 2016;52:7596–7599. - PubMed
- Berger A. L., Donabauer K., König B. Chem. Sci. 2018;9:7230–7235. - PMC - PubMed
- Ding W., Lu L.-Q., Liu J., Liu D., Song H.-T., Xiao W.-J. J. Org. Chem. 2016;81:7237–7243. - PubMed
- Petronijević F. R., Nappi M., MacMillan D. W. C. J. Am. Chem. Soc. 2013;135:18323–18326. - PMC - PubMed
- Du J., Skubi K. L., Schultz D. M., Yoon T. P. Science. 2014;344:392–396. - PMC - PubMed
- Rono L. J., Yayla H. G., Wang D. Y., Armstrong M. F., Knowles R. R. J. Am. Chem. Soc. 2013;135:17735–17738. - PubMed
- Nakajima M., Fava E., Loescher S., Jiang Z., Rueping M. Angew. Chem., Int. Ed. 2015;54:8828–8832. - PubMed
-
-
- Roth H. G., Romero N. A., Nicewicz D. A. Synlett. 2016;27:714–723.
-
-
For selected examples, see:
- Clerici A., Porta O., Zago P. Tetrahedron. 1986;42:561–572.
- Clerici A., Porta O. J. Org. Chem. 1989;54:3872–3878.
-
Publication types
LinkOut - more resources
Full Text Sources

