A macrocyclic oligofuran: synthesis, solid state structure and electronic properties
- PMID: 32055302
- PMCID: PMC7003964
- DOI: 10.1039/c9sc03247a
A macrocyclic oligofuran: synthesis, solid state structure and electronic properties
Abstract
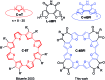
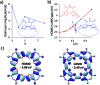
We report the first π-conjugated macrocyclic system with an oligofuran backbone. The calculated HOMO-LUMO gap is similar to that of the corresponding linear polymer, indicating a remarkable electron delocalization. The X-ray structure reveals a planar conformation, in contrast to the twisted conformation of macrocyclic oligothiophenes. The intermolecular π-π stacking distance is extremely small (3.17 Å), indicating very strong interactions. The macrocycle forms large π-aggregates in solution and shows a tendency toward highly ordered multilayer adsorption at the solid-liquid interface. The face-on orientation of molecules explains the higher hole mobility observed in the out-of-plane direction.
This journal is © The Royal Society of Chemistry 2019.
Figures
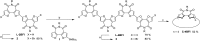





References
-
- Iyoda M., Yamakawa J., Rahman M. J. Angew. Chem., Int. Ed. 2011;50:10522–10553. - PubMed
-
- Xu Y., Kaur R., Wang B., Minameyer M. B., Gsänger S., Meyer B., Drewello T., Guldi D. M., von Delius M. J. Am. Chem. Soc. 2018;140:13413–13420. - PubMed
-
- Zhong Y., Yang Y., Shen Y., Xu W., Wang Q., Connor A. L., Zhou X., He L., Zeng X. C., Shao Z., Lu Z.-l., Gong B. J. Am. Chem. Soc. 2017;139:15950–15957. - PubMed
-
- Lorbach D., Keerthi A., Figueira-Duarte T. M., Baumgarten M., Wagner M., Müllen K. Angew. Chem., Int. Ed. 2016;55:418–421. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

