Highly stereoselective nickel-catalyzed difluoroalkylation of aryl ketones to tetrasubstituted monofluoroalkenes and quaternary alkyl difluorides
- PMID: 32055314
- PMCID: PMC7003883
- DOI: 10.1039/c9sc02806d
Highly stereoselective nickel-catalyzed difluoroalkylation of aryl ketones to tetrasubstituted monofluoroalkenes and quaternary alkyl difluorides
Abstract
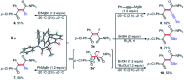
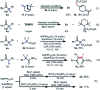
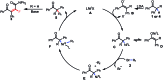
A nickel-catalyzed difluoroalkylation of α-C-H bonds of aryl ketones to furnish highly stereo-defined tetrasubstituted monofluoroalkenes or quaternary alkyl difluorides from secondary or tertiary ketones, respectively, has been established. Mechanistic investigations indicated that these C-H fluoroalkylations proceed via a Ni(i)/Ni(iii) catalytic cycle. An obvious fluorine effect was observed in the reaction, and this reaction has demonstrated high stereoselectivity, mild conditions, and broad substrate scopes, thus enabling the late-stage fluoroalkylation of bioactive molecules. This method offers a solution for expedient construction of monofluoroalkenes from readily available materials, and provides an efficient approach for the synthesis of bioactive fluorinated compounds for the discovery of lead compounds in medicinal chemistry.
This journal is © The Royal Society of Chemistry 2019.
Figures




Similar articles
-
Cobalt-catalyzed difluoroalkylation of tertiary aryl ketones for facile synthesis of quaternary alkyl difluorides.Nat Commun. 2018 Nov 23;9(1):4951. doi: 10.1038/s41467-018-07525-y. Nat Commun. 2018. PMID: 30470757 Free PMC article.
-
Blue Light Promoted Difluoroalkylation of Aryl Ketones: Synthesis of Quaternary Alkyl Difluorides and Tetrasubstituted Monofluoroalkenes.Org Lett. 2020 Jun 5;22(11):4261-4265. doi: 10.1021/acs.orglett.0c01294. Epub 2020 May 14. Org Lett. 2020. PMID: 32407104
-
Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides.Acc Chem Res. 2018 Sep 18;51(9):2264-2278. doi: 10.1021/acs.accounts.8b00230. Epub 2018 Aug 22. Acc Chem Res. 2018. PMID: 30132322
-
Transition metal-catalyzed C(sp2/sp3)-H α-fluoroalkenylation from gem-(bromo/di)fluoroalkenes to monofluoroalkenes: scope, mechanisms, and synthetic applications.Org Biomol Chem. 2024 Aug 28;22(34):6860-6904. doi: 10.1039/d4ob01044b. Org Biomol Chem. 2024. PMID: 39136141 Review.
-
[Development of highly stereoselective reactions utilizing heteroatoms--new approach to the stereoselective Horner-Wadsworth-Emmons reaction].Yakugaku Zasshi. 2000 May;120(5):432-44. doi: 10.1248/yakushi1947.120.5_432. Yakugaku Zasshi. 2000. PMID: 10825807 Review. Japanese.
Cited by
-
On the application of 3d metals for C-H activation toward bioactive compounds: The key step for the synthesis of silver bullets.Beilstein J Org Chem. 2021 Jul 30;17:1849-1938. doi: 10.3762/bjoc.17.126. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34386103 Free PMC article. Review.
-
Cobalt-catalyzed decarboxylative difluoroalkylation of nitrophenylacetic acid salts.Chem Sci. 2023 Nov 16;14(47):13902-13907. doi: 10.1039/d3sc05583c. eCollection 2023 Dec 6. Chem Sci. 2023. PMID: 38075641 Free PMC article.
-
Vessel effects in organic chemical reactions; a century-old, overlooked phenomenon.Chem Sci. 2022 May 4;13(21):6181-6196. doi: 10.1039/d2sc01125e. eCollection 2022 Jun 1. Chem Sci. 2022. PMID: 35733904 Free PMC article. Review.
-
Recent Trends in Photocatalytic Enantioselective Reactions.Top Curr Chem (Cham). 2022 Sep 16;380(6):48. doi: 10.1007/s41061-022-00402-9. Top Curr Chem (Cham). 2022. PMID: 36112295 Review.
References
-
- Levenson A. S., Jordan V. C. Eur. J. Cancer. 1999;35:1974. - PubMed
- McCague R., Leclercq G., Legros N., Goodman J., Blackburn G. M., Jarman M., Foster A. B. J. Med. Chem. 1989;32:2527. - PubMed
- Connor C. E., Norris J. D., Broadwater G., Willson T. M., Gottardis M. M., Dewhirst M. W., McDonnell D. P. Cancer Res. 2001;61:2917. - PubMed
- Oishi S., Miyamoto K., Niida A., Yamamoto M., Ajito K., Tamamura H., Otaka A., Kuroda Y., Asai A., Fujii N. Tetrahedron. 2006;62:1416.
-
- Schreivogel A., Maurer J., Winter R., Baro A., Laschat S. Eur. J. Org. Chem. 2006;2006:3395.
- Schultz A., Laschat S., Diele S., Nimtz M. Eur. J. Org. Chem. 2003;2003:2829.
- Babudri F., Farinola G. M., Naso F., Ragni R. Chem. Commun. 2007:1003. - PubMed
-
- Hirai G., Nishizawa E., Kakumoto D., Morita M., Okada M., Hashizume D., Nagashima S., Sodeoka M. Chem. Lett. 2015;44:1389.
- Lei X. S., Dutheuil G., Pannecoucke X., Quirion J. C. Org. Lett. 2004;6:2101. - PubMed
- Burton D. J., Yang Z.-Y., Qiu W. Chem. Rev. 1996;96:1641. - PubMed
- Dutheuil G., Pierry C., Villiers E., Couve-Bonnaire S., Pannecoucke X. New J. Chem. 2013;37:1320.
- Depré D., Vermeulen W. A. A., Lang Y., Dubois J., Vandevivere J., Vandermeersch J., Huang L., Robiette R. Org. Lett. 2017;19:1414. - PubMed
- Liu Y., Zhang K., Huang Y., Pan S., Liu X.-Q., Yang Y., Jiang Y., Xu X.-H. Chem. Commun. 2016;52:5969. - PubMed
- Liao F.-M., Cao Z.-Y., Yu J.-S., Zhou J. Angew. Chem., Int. Ed. 2017;56:2459. - PubMed
LinkOut - more resources
Full Text Sources

