Chemical communication in spatially organized protocell colonies and protocell/living cell micro-arrays
- PMID: 32055320
- PMCID: PMC6991169
- DOI: 10.1039/c9sc04522h
Chemical communication in spatially organized protocell colonies and protocell/living cell micro-arrays
Abstract
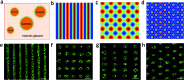
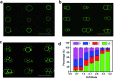
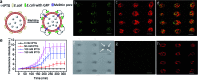
Micro-arrays of discrete or hemifused giant unilamellar lipid vesicles (GUVs) with controllable spatial geometries, lattice dimensions, trapped occupancies and compositions are prepared by acoustic standing wave patterning, and employed as platforms to implement chemical signaling in GUV colonies and protocell/living cell consortia. The methodology offers an alternative approach to GUV micro-array fabrication and provides new opportunities in protocell research and bottom-up synthetic biology.
This journal is © The Royal Society of Chemistry 2019.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

