Chronic exposure to complex metal oxide nanoparticles elicits rapid resistance in Shewanella oneidensis MR-1
- PMID: 32055346
- PMCID: PMC6993611
- DOI: 10.1039/c9sc01942a
Chronic exposure to complex metal oxide nanoparticles elicits rapid resistance in Shewanella oneidensis MR-1
Abstract
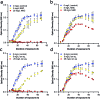
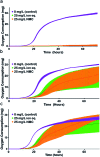
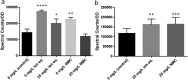
Engineered nanoparticles are incorporated into numerous emerging technologies because of their unique physical and chemical properties. Many of these properties facilitate novel interactions, including both intentional and accidental effects on biological systems. Silver-containing particles are widely used as antimicrobial agents and recent evidence indicates that bacteria rapidly become resistant to these nanoparticles. Much less studied is the chronic exposure of bacteria to particles that were not designed to interact with microorganisms. For example, previous work has demonstrated that the lithium intercalated battery cathode nanosheet, nickel manganese cobalt oxide (NMC), is cytotoxic and causes a significant delay in growth of Shewanella oneidensis MR-1 upon acute exposure. Here, we report that S. oneidensis MR-1 rapidly adapts to chronic NMC exposure and is subsequently able to survive in much higher concentrations of these particles, providing the first evidence of permanent bacterial resistance following exposure to nanoparticles that were not intended as antibacterial agents. We also found that when NMC-adapted bacteria were subjected to only the metal ions released from this material, their specific growth rates were higher than when exposed to the nanoparticle. As such, we provide here the first demonstration of bacterial resistance to complex metal oxide nanoparticles with an adaptation mechanism that cannot be fully explained by multi-metal adaptation. Importantly, this adaptation persists even after the organism has been grown in pristine media for multiple generations, indicating that S. oneidensis MR-1 has developed permanent resistance to NMC.
This journal is © The Royal Society of Chemistry 2019.
Figures





Similar articles
-
Effect of metal cations on antimicrobial activity and compartmentalization of silver in Shewanella oneidensis MR-1 upon exposure to silver ions.Sci Total Environ. 2022 Sep 10;838(Pt 3):156401. doi: 10.1016/j.scitotenv.2022.156401. Epub 2022 May 30. Sci Total Environ. 2022. PMID: 35654200
-
A mechanistic study of TiO2 nanoparticle toxicity on Shewanella oneidensis MR-1 with UV-containing simulated solar irradiation: Bacterial growth, riboflavin secretion, and gene expression.Chemosphere. 2017 Feb;168:1158-1168. doi: 10.1016/j.chemosphere.2016.10.085. Epub 2016 Nov 4. Chemosphere. 2017. PMID: 27823777
-
MgtE Homolog FicI Acts as a Secondary Ferrous Iron Importer in Shewanella oneidensis Strain MR-1.Appl Environ Microbiol. 2018 Mar 1;84(6):e01245-17. doi: 10.1128/AEM.01245-17. Print 2018 Mar 15. Appl Environ Microbiol. 2018. PMID: 29330185 Free PMC article.
-
[Advances in electrochemically active biofilm of Shewanella oneidensis MR-1].Sheng Wu Gong Cheng Xue Bao. 2023 Mar 25;39(3):881-897. doi: 10.13345/j.cjb.220468. Sheng Wu Gong Cheng Xue Bao. 2023. PMID: 36994560 Review. Chinese.
-
A Review on Nano-Antimicrobials: Metal Nanoparticles, Methods and Mechanisms.Curr Drug Metab. 2017;18(2):120-128. doi: 10.2174/1389200217666161201111146. Curr Drug Metab. 2017. PMID: 27908256 Review.
Cited by
-
Metallic Nanoparticles-Friends or Foes in the Battle against Antibiotic-Resistant Bacteria?Microorganisms. 2021 Feb 12;9(2):364. doi: 10.3390/microorganisms9020364. Microorganisms. 2021. PMID: 33673231 Free PMC article. Review.
-
Targeted nanomedicines for the treatment of Helicobacter pylori infection.Mater Today Bio. 2025 Apr 30;32:101820. doi: 10.1016/j.mtbio.2025.101820. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40458694 Free PMC article. Review.
-
Environmentally relevant concentrations of titanium dioxide nanoparticles pose negligible risk to marine microbes.Environ Sci Nano. 2021 Apr 9;8(5):1236-1255. doi: 10.1039/d0en00883d. Environ Sci Nano. 2021. PMID: 34046180 Free PMC article.
-
Nanoscale battery cathode materials induce DNA damage in bacteria.Chem Sci. 2020 Sep 21;11(41):11244-11258. doi: 10.1039/d0sc02987d. Chem Sci. 2020. PMID: 34094365 Free PMC article.
-
Bactericidal Antibacterial Mechanism of Plant Synthesized Silver, Gold and Bimetallic Nanoparticles.Pharmaceutics. 2020 Oct 30;12(11):1044. doi: 10.3390/pharmaceutics12111044. Pharmaceutics. 2020. PMID: 33143388 Free PMC article. Review.
References
-
- Benn T. M., Westerhoff P. Environ. Sci. Technol. 2008;42:4133–4139. - PubMed
-
- Lankone R. S., Challis K. E., Bi Y., Hanigan D., Reed R. B., Zaikova T., Hutchison J. E., Westerhoff P., Ranville J., Fairbrother H., Gilbertson L. M. Environ. Sci.: Nano. 2017;4:1784–1797.
-
- Talapin D. V., Steckel J. MRS Bull. 2013;38:685–691.
-
- Stefaniuk M., Oleszczuk P., Ok Y. S. Chem. Eng. J. 2016;287:618–632.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

