Chiroptical inversion of a planar chiral redox-switchable rotaxane
- PMID: 32055357
- PMCID: PMC7003955
- DOI: 10.1039/c9sc03694f
Chiroptical inversion of a planar chiral redox-switchable rotaxane
Abstract
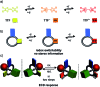
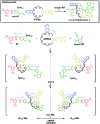
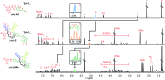
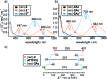
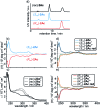
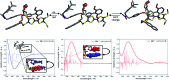
A tetrathiafulvalene (TTF)-containing crown ether macrocycle with C s symmetry was designed to implement planar chirality into a redox-active [2]rotaxane. The directionality of the macrocycle atom sequence together with the non-symmetric axle renders the corresponding [2]rotaxane mechanically planar chiral. Enantiomeric separation of the [2]rotaxane was achieved by chiral HPLC. The electrochemical properties - caused by the reversible oxidation of the TTF - are similar to a non-chiral control. Reversible inversion of the main band in the ECD spectra for the individual enantiomers was observed after oxidation. Experimental evidence, conformational analysis and DFT calculations of the neutral and doubly oxidised species indicate that mainly electronic effects of the oxidation are responsible for the chiroptical switching. This is the first electrochemically switchable rotaxane with a reversible inversion of the main ECD band.
This journal is © The Royal Society of Chemistry 2019.
Figures
References
LinkOut - more resources
Full Text Sources

