Orexin-A aggravates cytotoxicity and mitochondrial impairment in SH-SY5Y cells transfected with APPswe via p38 MAPK pathway
- PMID: 32055596
- PMCID: PMC6995740
- DOI: 10.21037/atm.2019.11.68
Orexin-A aggravates cytotoxicity and mitochondrial impairment in SH-SY5Y cells transfected with APPswe via p38 MAPK pathway
Abstract
Background: Alzheimer's disease (AD) is one of the common neurodegenerative diseases and is characterized by the accumulation of amyloid-β (Aβ). Orexin-A is a neuropeptide produced in the hypothalamus and thought to be involved in the pathogenesis of AD. However, its underlying mechanism and signaling pathway remains unclear. The aim of this work was to investigate the effect of Orexin-A on AD, and to explore its potential mechanism and signaling pathway.
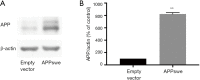
Methods: SH-SY5Y cells that were stably transfected with the Swedish mutant amyloid precursor protein (APPswe), a cell model of AD with excessive Aβ production, were used in this study. Cells were treated with Orexin-A, and with or without SB203580, an inhibitor of the p38 mitogen-activated protein kinase (MAPK) pathway, one of the key MAPK pathways associated with cell death. Following treatment, cells were collected and analyzed by western blotting, ELISA, electron microscopy, real-time PCR, fluorescence microscopy, and other biochemical assays.
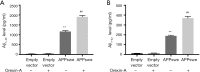
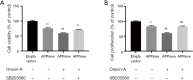
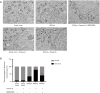
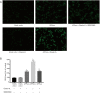
Results: Orexin-A increased the level of Aβ1-40 and Aβ1-42 in the cell medium, and activated the p38 MAPK pathway. As evidenced by the CCK-8 and ELISA BrdU assays, Orexin-A decreased cell viability and cell proliferation. Electron microscopic analysis used to observe the morphology of mitochondria, showed that Orexin-A increased the percentage of abnormal mitochondria. Further, decreased activity of cytochrome c oxidase (CCO), level of ATP, and mitochondrial DNA (mtDNA) copy number following Orexin-A treatment showed that Orexin-A exacerbated mitochondrial dysfunction. The level of intracellular reactive oxygen species (ROS), which is mainly generated in mitochondria and reflects mitochondrial dysfunction, was also increased by Orexin-A. SB203580 blocked the cytotoxicity and mitochondrial impairment aggravated by Orexin-A.
Conclusions: These findings demonstrate that Orexin-A aggravates cytotoxicity and mitochondrial impairment in SH-SY5Y cells transfected with APPswe through the p38 MAPK pathway, and suggest that Orexin-A participates in the pathogenesis of AD, which may provide a new treatment target in the future.
Keywords: Alzheimer’s disease (AD); Orexin-A; cytotoxicity; mitochondrial impairment; p38 MAPK pathway.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflict of interest to declare.
Figures


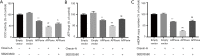
References
LinkOut - more resources
Full Text Sources
Research Materials
