Proteus mirabilis outcompetes Klebsiella pneumoniae in artificial urine medium through secretion of ammonia and other volatile compounds
- PMID: 32055744
- PMCID: PMC7005574
- DOI: 10.1016/j.heliyon.2020.e03361
Proteus mirabilis outcompetes Klebsiella pneumoniae in artificial urine medium through secretion of ammonia and other volatile compounds
Abstract
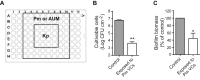
Klebsiella pneumoniae and Proteus mirabilis form mixed biofilms in catheter-associated urinary tract infections. However, co-inoculation of P. mirabilis with K. pneumoniae in artificial urine medium (AUM) resulted in a drastic reduction of K. pneumoniae cells in both biofilm and planktonic growth. Here, the mechanism behind this competitive interaction was studied. Both pH and aqueous ammonia (NH3aq) increased in mixed cultures (to 9.3 and 150 mM, respectively), while K. pneumoniae viable cells dramatically diminished over time (>6-log reduction, p < 0.05). Mixed cultures developed in either 2-(N-morpholino) ethanesulfonic acid (MES)-buffered AUM (pH 6.5) or AUM without urea did not show bacterial competition, evidencing that the increase in pH and/or NH3aq concentration play a role in the competitive interaction. Viability of K. pneumoniae single-species cultures decreased 1.5-log in alkaline AUM containing 150 mM NH3aq after 24 h inoculation, suggesting that ammonia is involved in this inter-species competition. Besides NH3aq, additional antimicrobials should be present to get the whole competitive effect. Supernatants from P. mirabilis-containing cultures significantly diminished K. pneumoniae viability in planktonic cultures and affected biofilm biomass (p < 0.05). When subjected to evaporation, these supernatants lost their antimicrobial activity suggesting the volatile nature of the antimicrobial compounds. Exposure of K. pneumoniae to volatile compounds released by P. mirabilis significantly decreased cell viability in both planktonic and biofilm cultures (p < 0.05). The current investigation also evidenced a similar bactericidal effect of P. mirabilis volatiles over Escherichia coli and Morganella morganii. Altogether, these results evidence the secretion of ammonia and other volatile compounds by P. mirabilis, with antimicrobial activity against gram-negative uropathogens including K. pneumoniae. This investigation provides novel insight into competitive inter-species interactions that are mediated by production of volatile molecules.
Keywords: Artificial urine medium; Bacteria; Biofilms; Dual-species competition; Klebsiella pneumoniae; Metabolite; Microbiology; Proteus mirabilis; Volatile compounds.
© 2020 The Authors.
Figures




Similar articles
-
Species interactions in mixed-community crystalline biofilms on urinary catheters.J Med Microbiol. 2007 Nov;56(Pt 11):1549-1557. doi: 10.1099/jmm.0.47395-0. J Med Microbiol. 2007. PMID: 17965358
-
Role of interspecies interactions in dual-species biofilms developed in vitro by uropathogens isolated from polymicrobial urinary catheter-associated bacteriuria.Biofouling. 2016 Oct;32(9):1067-77. doi: 10.1080/08927014.2016.1231300. Biofouling. 2016. PMID: 27642801
-
Role of nutrient limitation in the competition between uropathogenic strains of Klebsiella pneumoniae and Escherichia coli in mixed biofilms.Biofouling. 2018 Mar;34(3):287-298. doi: 10.1080/08927014.2018.1434876. Epub 2018 Feb 19. Biofouling. 2018. PMID: 29457734
-
Proteus mirabilis and Klebsiella pneumoniae as pathogens capable of causing co-infections and exhibiting similarities in their virulence factors.Front Cell Infect Microbiol. 2022 Oct 20;12:991657. doi: 10.3389/fcimb.2022.991657. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36339335 Free PMC article. Review.
-
Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done.J Intern Med. 2014 Aug;276(2):120-9. doi: 10.1111/joim.12220. J Intern Med. 2014. PMID: 24635559 Review.
Cited by
-
Why Do These Yeasts Smell So Good? Volatile Organic Compounds (VOCs) Produced by Malassezia Species in the Exponential and Stationary Growth Phases.Molecules. 2023 Mar 14;28(6):2620. doi: 10.3390/molecules28062620. Molecules. 2023. PMID: 36985592 Free PMC article.
-
Pangenome Analysis Reveals Novel Contact-Dependent Growth Inhibition System and Phenazine Biosynthesis Operons in Proteus mirabilis BL95 That Are Located in An Integrative and Conjugative Element.Microorganisms. 2024 Jun 28;12(7):1321. doi: 10.3390/microorganisms12071321. Microorganisms. 2024. PMID: 39065090 Free PMC article.
-
Bacteria-Infected Artificial Urine Characterization Based on a Combined Approach Using an Electronic Tongue Complemented with 1H-NMR and Flow Cytometry.Biosensors (Basel). 2023 Oct 5;13(10):916. doi: 10.3390/bios13100916. Biosensors (Basel). 2023. PMID: 37887109 Free PMC article.
-
Hydrogen Sulfide and Carbon Monoxide Tolerance in Bacteria.Antioxidants (Basel). 2021 May 5;10(5):729. doi: 10.3390/antiox10050729. Antioxidants (Basel). 2021. PMID: 34063102 Free PMC article. Review.
-
Into the understanding the multicellular lifestyle of Proteus mirabilis on solid surfaces.Front Cell Infect Microbiol. 2022 Sep 2;12:864305. doi: 10.3389/fcimb.2022.864305. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36118021 Free PMC article. Review.
References
-
- Clarke K., Hall C.L., Wiley Z., Tejedor S.C., Kim J.S., Reif L., Witt L., Jacob J.T. Catheter-associated urinary tract infections in adults: diagnosis, treatment, and prevention. J. Hosp. Med. 2019;14:E1–E5. - PubMed
-
- Sabir N., Ikram A., Zaman G., Satti L., Gardezi A., Ahmed A., Ahmed P. Bacterial biofilm-based catheter-associated urinary tract infections: causative pathogens and antibiotic resistance. Am. J. Infect. Contr. 2017 - PubMed
-
- Azevedo A.S., Almeida C., Melo L.F., Azevedo N.F. Impact of polymicrobial biofilms in catheter-associated urinary tract infections. Crit. Rev. Microbiol. 2017;43:423–439. - PubMed
-
- Kotaskova I., Obrucova H., Malisova B., Videnska P., Zwinsova B., Peroutkova T., Dvorackova M., Kumstat P., Trojan P., Ruzicka F., Hola V., Freiberger T. Molecular techniques complement culture-based assessment of bacteria composition in mixed biofilms of urinary tract catheter-related samples. Front. Microbiol. 2019;10:462. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

