Extending varenicline preloading to 6 weeks facilitates smoking cessation: A single-site, randomised controlled trial
- PMID: 32055787
- PMCID: PMC7005428
- DOI: 10.1016/j.eclinm.2019.11.021
Extending varenicline preloading to 6 weeks facilitates smoking cessation: A single-site, randomised controlled trial
Abstract
Background: Initiating varenicline use 4 weeks before the target quit date (TQD) reduces smoking in the run-in phase and increases end-treatment cessation rates; however, the lack of a smoke intake plateau suggests longer preloading periods are required. This study assessed whether varenicline preloading for 6 weeks reduced pre-quit smoke intake and enhanced 6-month abstinence outcomes compared with the standard 1-week preloading.
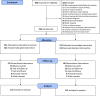
Methods: In this randomised single-centre controlled trial, (ClinicalTrials.gov identifier: NCT02634281), conducted between February 2016 and July 2018 in Israel, daily smokers (n = 242) aged ≥ 18 years were randomly assigned (1:1) to receive varenicline preloading for 6 weeks (n = 121) or a placebo for 5 weeks followed by varenicline for 1 week (n = 121) before the TQD. Participants and researchers were masked to both group assignment and treatment allocation. Both groups received standard 12-week post-TQD varenicline treatment. The primary outcome was the 24-week biochemically verified continuous abstinence rate (CAR) from weeks 6 (TQD)-30. Secondary outcomes included the 23-week CAR from 1-week post-TQD (week 7) to week 30, and the 7-day point-prevalence (PP) abstinence at week 30. Other measures included pre- and post-quit rewards, smoking urges, nausea, aversion, and markers of cigarette consumption.
Findings: By intention-to-treat, the 24-week CAR, weeks 6-30 with extended preloading was significantly higher than with standard preloading (23·1% vs. 4·1%; risk reduction [RR]: -0·19 [95% confidence interval [CI]:-0·10-0·24]; p < 0·001). Extended preloading also showed better secondary outcomes. Extended preloading significantly decreased pre-quit rewards, urges, and smoke intake, including unsolicited smoking abstinence. Post-quit urges remained remarkably lower with extended preloading. Participants receiving extended preloading reported more nausea at week 4 (39.6% vs 11.5%) and abnormal dreams at week 6 (7.7% vs. 0%). Participants receiving standard preloading reported more constipation at week 7 (7.6% vs. 0%) and dizziness at weeks 7 (12.1% vs. 2.5%) and 12 (10.7% vs 1.4%).
Interpretation: Extended preloading reduced ad lib smoking, enhanced cessation rates at 3 and 6 months, and decreased pre- and post-quit rewards and smoking drive in a pattern compatible with a reinforcement-reduction mechanism. These data substantiate extending the standard pre-treatment period, and suggest that targeting pre-quit smoking sensations should be a treatment priority, although confirmatory evidence is needed from larger clinical trials.
Funding: This study was funded by a 2013 Global Research Award for Nicotine Dependence (GRAND) supported by Pfizer, Inc. (#WI182915).
Keywords: Extended preloading; Smoking cessation; Smoking reduction; Varenicline.
© 2019 Published by Elsevier Ltd.
Conflict of interest statement
None.
Figures

References
-
- Report of the Surgeon General: How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease, 2010: The Report Available at: http://www.surgeongeneral.gov/library/reports/tobaccosmoke/index.html. - PubMed
-
- Fiore MC, Jaén CR, Baker TB. Treating tobacco use and dependence: 2008 update. US Depart Health Hum Serv. 2008
-
- Hajek P., McRobbie H.J., Myers K. Use of varenicline for 4 weeks before quitting smoking. Arch Intern Med. 2011;171:770–777. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

