Exploration of databases and methods supporting drug repurposing: a comprehensive survey
- PMID: 32055842
- PMCID: PMC7986597
- DOI: 10.1093/bib/bbaa003
Exploration of databases and methods supporting drug repurposing: a comprehensive survey
Abstract
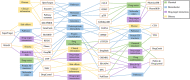
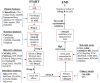
Drug development involves a deep understanding of the mechanisms of action and possible side effects of each drug, and sometimes results in the identification of new and unexpected uses for drugs, termed as drug repurposing. Both in case of serendipitous observations and systematic mechanistic explorations, confirmation of new indications for a drug requires hypothesis building around relevant drug-related data, such as molecular targets involved, and patient and cellular responses. These datasets are available in public repositories, but apart from sifting through the sheer amount of data imposing computational bottleneck, a major challenge is the difficulty in selecting which databases to use from an increasingly large number of available databases. The database selection is made harder by the lack of an overview of the types of data offered in each database. In order to alleviate these problems and to guide the end user through the drug repurposing efforts, we provide here a survey of 102 of the most promising and drug-relevant databases reported to date. We summarize the target coverage and types of data available in each database and provide several examples of how multi-database exploration can facilitate drug repurposing.
Keywords: biomolecular databases; disease databases; drug databases; drug repositioning; drug–target interaction databases.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Shah NP, Tran C, Lee FY, et al. . Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004;305:399–401. - PubMed
-
- Paul SM, Mytelka DS, Dunwiddie CT, et al. . How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov 2010;9:203–14. - PubMed
-
- Dickson M, Gagnon JP. The cost of new drug discovery and development. Discov Med 2009;4:172–9. - PubMed
-
- Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med 2017;281:365–82. - PubMed
-
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med 1980;303:1480–1. - PubMed

