Sleep Deprivation Affects Tau Phosphorylation in Human Cerebrospinal Fluid
- PMID: 32057125
- PMCID: PMC7289061
- DOI: 10.1002/ana.25702
Sleep Deprivation Affects Tau Phosphorylation in Human Cerebrospinal Fluid
Abstract
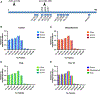
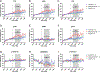
Tau hyperphosphorylation is an early step in tau-mediated neurodegeneration and is associated with intracellular aggregation of tau as neurofibrillary tangles, neuronal and synaptic loss, and eventual cognitive dysfunction in Alzheimer disease. Sleep loss increases the cerebrospinal fluid concentration of amyloid-β and tau. Using mass spectrometry, we measured tau and phosphorylated tau concentrations in serial samples of cerebrospinal fluid collected from participants who were sleep-deprived, treated with sodium oxybate, or allowed to sleep normally. We found that sleep loss affected phosphorylated tau differently depending on the modified site. These findings suggest a mechanism for sleep loss to increase risk of Alzheimer disease. ANN NEUROL 2020;87:700-709.
© 2020 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest:
Washington University, with N.R.B. and R.J.B. as coinventors, has submitted the U.S. nonprovisional patent application “Cerebrospinal fluid (CSF) Tau Rate of Phosphorylation Measurement to Define Stages of Alzheimer’s Disease and Monitor Brain Kinases/Phosphatases Activity.”
Figures
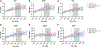
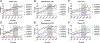
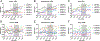
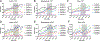

References
-
- Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Molec Neurosci 2001; 17 (2): 101–18. - PubMed
-
- Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol 2013; 12 (6): 609–22. - PubMed
-
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 2005; 48: 913–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

