Functional heterogeneity of lymphocytic patterns in primary melanoma dissected through single-cell multiplexing
- PMID: 32057296
- PMCID: PMC7053517
- DOI: 10.7554/eLife.53008
Functional heterogeneity of lymphocytic patterns in primary melanoma dissected through single-cell multiplexing
Abstract
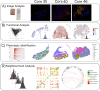
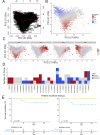
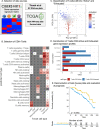
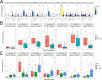
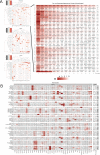
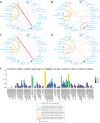
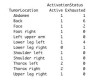
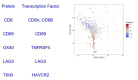
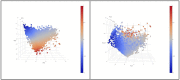
In melanoma, the lymphocytic infiltrate is a prognostic parameter classified morphologically into 'brisk', 'non-brisk' and 'absent' entailing a functional association that has never been proved. Recently, it has been shown that lymphocytic populations can be very heterogeneous, and that anti-PD-1 immunotherapy supports activated T cells. Here, we characterize the immune landscape in primary melanoma by high-dimensional single-cell multiplex analysis in tissue sections (MILAN technique) followed by image analysis, RT-PCR and shotgun proteomics. We observed that the brisk and non-brisk patterns are heterogeneous functional categories that can be further sub-classified into active, transitional or exhausted. The classification of primary melanomas based on the functional paradigm also shows correlation with spontaneous regression, and an improved prognostic value when compared to that of the brisk classification. Finally, the main inflammatory cell subpopulations that are present in the microenvironment associated with activation and exhaustion and their spatial relationships are described using neighbourhood analysis.
Keywords: TILs; brisk; cancer biology; human; melanoma; microenvironment; multiplex; tumor-infiltrating lymphocytes.
© 2020, Bosisio et al.
Conflict of interest statement
FB, Yv, LM, CC, JW, FM, MS, VB, OB, GC, Jv No competing interests declared, AA Affiliated with ProtATonce Ltd. The author has no other competing interests to declare, MB Has received funding from GlaxoSmithKline. The author has no other competing interests to declare, LA Affiliated with ProtATonce Ltd
Figures















References
-
- A-Gonzalez N, Quintana JA, García-Silva S, Mazariegos M, González de la Aleja A, Nicolás-Ávila JA, Walter W, Adrover JM, Crainiciuc G, Kuchroo VK, Rothlin CV, Peinado H, Castrillo A, Ricote M, Hidalgo A. Phagocytosis imprints heterogeneity in tissue-resident macrophages. The Journal of Experimental Medicine. 2017;214:1281–1296. doi: 10.1084/jem.20161375. - DOI - PMC - PubMed
-
- Ascierto PA, Agarwala SS, Ciliberto G, Demaria S, Dummer R, Duong CPM, Ferrone S, Formenti SC, Garbe C, Halaban R, Khleif S, Luke JJ, Mir LM, Overwijk WW, Postow M, Puzanov I, Sondel P, Taube JM, Thor Straten P, Stroncek DF, Wargo JA, Zarour H, Thurin M. Future perspectives in melanoma research "Melanoma Bridge", Napoli, November 30th-3rd December 2016. Journal of Translational Medicine. 2017;15:236. doi: 10.1186/s12967-017-1341-2. - DOI - PMC - PubMed
-
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, Piha-Paul SA, Yearley J, Seiwert TY, Ribas A, McClanahan TK. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. Journal of Clinical Investigation. 2017;127:2930–2940. doi: 10.1172/JCI91190. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

