Targeting Hsc70-based autophagy to eliminate amyloid β oligomers
- PMID: 32057360
- PMCID: PMC7085976
- DOI: 10.1016/j.bbrc.2020.02.016
Targeting Hsc70-based autophagy to eliminate amyloid β oligomers
Abstract
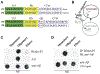
Amyloid β (Aβ) oligomers may be a real culprit in the pathogenesis of Alzheimer's disease (AD); therefore, the elimination of these toxic oligomers may be of great significance for AD therapy. Autophagy is the catabolic process by which lysosomes degrade cytosolic components, and heat shock cognate 70 kDa protein (Hsc70) binds to proteins with their KFERQ-like motifs [also known as chaperone-mediated autophagy (CMA) motifs] and carries them to lysosomes through CMA or late endosomes through endosomal microautophagy (eMI) for degradation. In this study, our strategy is to make the pathological Aβ become one selective and suitable substrate for CMA and eMI (termed as Hsc70-based autophagy) by tagging its oligomers with multiple CMA motifs. First, we design and synthesize Aβ oligomer binding peptides with three CMA motifs. Second, we determine that the peptide can help Aβ oligomers enter endosomes and lysosomes, which can be further enhanced by ketone. More importantly, we find that the peptide can dramatically reduce Aβ oligomers in induced pluripotent stem cell (iPSC) cortical neurons derived from AD patient fibroblasts and protect primary cultured cortical neurons against the Aβ oligomer-induced neurotoxicity. In conclusion, we demonstrate that the peptide targeting Hsc70-based autophagy can effectively eliminate Aβ oligomers and have superior neuroprotective activity.
Keywords: Alzheimer’s disease; Aβ oligomers; Chaperone-mediated autophagy; Hsc70.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


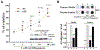
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

