Computational modeling of cardiac growth and remodeling in pressure overloaded hearts-Linking microstructure to organ phenotype
- PMID: 32058078
- PMCID: PMC7311197
- DOI: 10.1016/j.actbio.2020.02.010
Computational modeling of cardiac growth and remodeling in pressure overloaded hearts-Linking microstructure to organ phenotype
Abstract
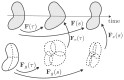
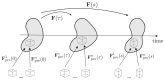
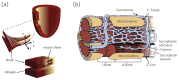
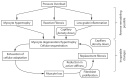
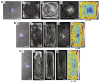
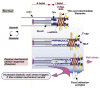
Cardiac growth and remodeling (G&R) refers to structural changes in myocardial tissue in response to chronic alterations in loading conditions. One such condition is pressure overload where elevated wall stresses stimulate the growth in cardiomyocyte thickness, associated with a phenotype of concentric hypertrophy at the organ scale, and promote fibrosis. The initial hypertrophic response can be considered adaptive and beneficial by favoring myocyte survival, but over time if pressure overload conditions persist, maladaptive mechanisms favoring cell death and fibrosis start to dominate, ultimately mediating the transition towards an overt heart failure phenotype. The underlying mechanisms linking biological factors at the myocyte level to biomechanical factors at the systemic and organ level remain poorly understood. Computational models of G&R show high promise as a unique framework for providing a quantitative link between myocardial stresses and strains at the organ scale to biological regulatory processes at the cellular level which govern the hypertrophic response. However, microstructurally motivated, rigorously validated computational models of G&R are still in their infancy. This article provides an overview of the current state-of-the-art of computational models to study cardiac G&R. The microstructure and mechanosensing/mechanotransduction within cells of the myocardium is discussed and quantitative data from previous experimental and clinical studies is summarized. We conclude with a discussion of major challenges and possible directions of future research that can advance the current state of cardiac G&R computational modeling. STATEMENT OF SIGNIFICANCE: The mechanistic links between organ-scale biomechanics and biological factors at the cellular size scale remain poorly understood as these are largely elusive to investigations using experimental methodology alone. Computational G&R models show high promise to establish quantitative links which allow more mechanistic insight into adaptation mechanisms and may be used as a tool for stratifying the state and predict the progression of disease in the clinic. This review provides a comprehensive overview of research in this domain including a summary of experimental data. Thus, this study may serve as a basis for the further development of more advanced G&R models which are suitable for making clinical predictions on disease progression or for testing hypotheses on pathogenic mechanisms using in-silico models.
Keywords: Computational modeling; Growth and remodeling; Hypertrophy; Pressure overload; Structural remodeling.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Esposito G, Rapacciuolo A, Prasad SVN, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

