Role of 1-Deoxysphingolipids in docetaxel neurotoxicity
- PMID: 32058598
- PMCID: PMC7426245
- DOI: 10.1111/jnc.14985
Role of 1-Deoxysphingolipids in docetaxel neurotoxicity
Abstract
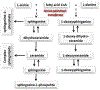
A major dose-limiting side effect of docetaxel chemotherapy is peripheral neuropathy. Patients' symptoms include pain, numbness, tingling and burning sensations, and motor weakness in the extremities. The molecular mechanism is currently not understood, and there are no treatments available. Previously, we have shown an association between neuropathy symptoms of patients treated with paclitaxel and the plasma levels of neurotoxic sphingolipids, the 1-deoxysphingolipids (1-deoxySL) (Kramer et al, FASEB J, 2015). 1-DeoxySL are produced when the first enzyme of the sphingolipid biosynthetic pathway, serine palmitoyltransferase (SPT), uses L-alanine as a substrate instead of its canonical amino acid substrate, L-serine. In the current investigation, we tested whether 1-deoxySL accumulate in the nervous system following systemic docetaxel treatment in mice. In dorsal root ganglia (DRG), we observed that docetaxel (45 mg/kg cumulative dose) significantly elevated the levels of 1-deoxySL and L-serine-derived ceramides, but not sphingosine-1-phosphate (S1P). S1P is a bioactive sphingolipid and a ligand for specific G-protein-coupled receptors. In the sciatic nerve, docetaxel decreased 1-deoxySL and ceramides. Moreover, we show that in primary DRG cultures, 1-deoxysphingosine produced neurite swellings that could be reversed with S1P. Our results demonstrate that docetaxel chemotherapy up-regulates sphingolipid metabolism in sensory neurons, leading to the accumulation of neurotoxic 1-deoxySL. We suggest that the neurotoxic effects of 1-deoxySL on axons can be reversed with S1P.
Keywords: 1-Deoxysphingolipids; Ceramide; Docetaxel-induced peripheral neuropathy; Serine palmitoyltransferase; Sphingosine-1-phosphate.
© 2020 International Society for Neurochemistry.
Conflict of interest statement
Figures





References
-
- Abram SE, Yi J, Fuchs A and Hogan QH (2006) Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology, 105, 146–153. - PubMed
-
- Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S and Kalofonos HP (2008) Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol, 66, 218–228. - PubMed
-
- Bhatnagar B, Gilmore S, Goloubeva O, Pelser C, Medeiros M, Chumsri S, Tkaczuk K, Edelman M and Bao T (2014) Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus, 3, 366. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

