Clinical Trial Generalizability Assessment in the Big Data Era: A Review
- PMID: 32058639
- PMCID: PMC7359942
- DOI: 10.1111/cts.12764
Clinical Trial Generalizability Assessment in the Big Data Era: A Review
Abstract
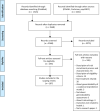
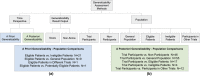
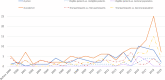
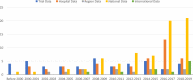
Clinical studies, especially randomized, controlled trials, are essential for generating evidence for clinical practice. However, generalizability is a long-standing concern when applying trial results to real-world patients. Generalizability assessment is thus important, nevertheless, not consistently practiced. We performed a systematic review to understand the practice of generalizability assessment. We identified 187 relevant articles and systematically organized these studies in a taxonomy with three dimensions: (i) data availability (i.e., before or after trial (a priori vs. a posteriori generalizability)); (ii) result outputs (i.e., score vs. nonscore); and (iii) populations of interest. We further reported disease areas, underrepresented subgroups, and types of data used to profile target populations. We observed an increasing trend of generalizability assessments, but < 30% of studies reported positive generalizability results. As a priori generalizability can be assessed using only study design information (primarily eligibility criteria), it gives investigators a golden opportunity to adjust the study design before the trial starts. Nevertheless, < 40% of the studies in our review assessed a priori generalizability. With the wide adoption of electronic health records systems, rich real-world patient databases are increasingly available for generalizability assessment; however, informatics tools are lacking to support the adoption of generalizability assessment practice.
© 2020 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

References
-
- From the NIH Director: the importance of clinical trials <http://www.nlm.nih.gov/medlineplus/magazine/issues/summer11/articles/sum...>. Accessed April 1, 2019.
-
- Sedgwick, P. External and internal validity in clinical trials. BMJ. 344, e1004 (2012).
-
- Beaver, J.A. , Ison, G. & Pazdur, R. Reevaluating eligibility criteria—balancing patient protection and participation in oncology trials. N. Engl. J. Med . 376, 1504–1505 (2017). - PubMed
-
- Shields, K.E. & Lyerly, A.D. Exclusion of pregnant women from industry‐sponsored clinical trials. Obstet. Gynecol. 122, 1077–1081 (2013). - PubMed
-
- US Food and Drug Administration . Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs guidance for industry <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents...> (2019). Accessed August 5, 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

