Improvement of cis, cis-Muconic Acid Production in Saccharomyces cerevisiae through Biosensor-Aided Genome Engineering
- PMID: 32058699
- PMCID: PMC8457548
- DOI: 10.1021/acssynbio.9b00477
Improvement of cis, cis-Muconic Acid Production in Saccharomyces cerevisiae through Biosensor-Aided Genome Engineering
Abstract
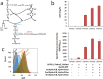
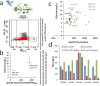
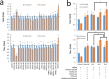
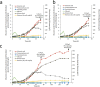
Muconic acid is a potential platform chemical for the production of nylon, polyurethanes, and terephthalic acid. It is also an attractive functional copolymer in plastics due to its two double bonds. At this time, no economically viable process for the production of muconic acid exists. To harness novel genetic targets for improved production of cis,cis-muconic acid (CCM) in the yeast Saccharomyces cerevisiae, we employed a CCM-biosensor coupled to GFP expression with a broad dynamic response to screen UV-mutagenesis libraries of CCM-producing yeast. Via fluorescence activated cell sorting we identified a clone Mut131 with a 49.7% higher CCM titer and 164% higher titer of biosynthetic intermediate-protocatechuic acid (PCA). Genome resequencing of the Mut131 and reverse engineering identified seven causal missense mutations of the native genes (PWP2, EST2, ATG1, DIT1, CDC15, CTS2, and MNE1) and a duplication of two CCM biosynthetic genes, encoding dehydroshikimate dehydratase and catechol 1,2-dioxygenase, which were not recognized as flux controlling before. The Mut131 strain was further rationally engineered by overexpression of the genes encoding for PCA decarboxylase and AROM protein without shikimate dehydrogenase domain (Aro1pΔE), and by restoring URA3 prototrophy. The resulting engineered strain produced 20.8 g/L CCM in controlled fed-batch fermentation, with a yield of 66.2 mg/g glucose and a productivity of 139 mg/L/h, representing the highest reported performance metrics in a yeast for de novo CCM production to date and the highest production of an aromatic compound in yeast. The study illustrates the benefit of biosensor-based selection and brings closer the prospect of biobased muconic acid.
Keywords: Saccharomyces cerevisiae; biosensor; muconic acid; mutagenesis; reverse engineering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Rorrer N. A.; Dorgan J. R.; Vardon D. R.; Martinez C. R.; Yang Y.; Beckham G. T. (2016) Renewable unsaturated polyesters from muconic acid. ACS Sustainable Chem. Eng. 4, 6867–6876. 10.1021/acssuschemeng.6b01820. - DOI
-
- van Duuren J. B.; Wijte D.; Leprince A.; Karge B.; Puchalka J.; Wery J.; Dos Santos V. A.; Eggink G.; Mars A. E. (2011) Generation of a catR deficient mutant of P. putida KT2440 that produces cis, cis-muconate from benzoate at high rate and yield. J. Biotechnol. 156, 163–172. 10.1016/j.jbiotec.2011.08.030. - DOI - PubMed
-
- Kohlstedt M.; Starck S.; Barton N.; Stolzenberger J.; Selzer M.; Mehlmann K.; Schneider R.; Pleissner D.; Rinkel J.; Dickschat J. S.; Venus J.; J B. J. H. v. D.; Wittmann C. (2018) From lignin to nylon: Cascaded chemical and biochemical conversion using metabolically engineered Pseudomonas putida. Metab. Eng. 47, 279–293. 10.1016/j.ymben.2018.03.003. - DOI - PubMed
-
- Vardon D. R.; Franden M. A.; Johnson C. W.; Karp E. M.; Guarnieri M. T.; Linger J. G.; Salm M. J.; Strathmann T. J.; Beckham G. T. (2015) Adipic acid production from lignin. Energy Environ. Sci. 8, 617–628. 10.1039/C4EE03230F. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

