Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study
- PMID: 32058843
- PMCID: PMC7280051
- DOI: 10.1200/JCO.19.02318
Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study
Abstract
Purpose: Trastuzumab deruxtecan (T-DXd, formerly DS-8201a) is a novel human epidermal growth factor receptor 2 (HER2)-targeted antibody drug conjugate (ADC) with a topoisomerase I inhibitor payload. A dose escalation and expansion phase I study evaluated the safety and activity of T-DXd in patients with advanced HER2-expressing/mutated solid tumors. Here, results for T-DXd at the recommended doses for expansion (RDE) in patients with HER2-low (immunohistochemistry [IHC] 1+ or IHC 2+/in situ hybridization-) breast cancer (ClinicalTrials.gov identifier: NCT02564900) are reported.
Patients and methods: Eligible patients had advanced/metastatic HER2-low-expressing breast cancer refractory to standard therapies. The RDE of 5.4 or 6.4 mg/kg T-DXd were administered intravenously once every 3 weeks until withdrawal of consent, unacceptable toxicity, or progressive disease. Antitumor activity and safety were assessed.
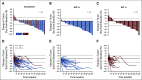
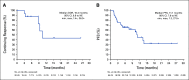
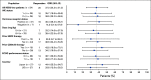
Results: Between August 2016 and August 2018, 54 patients were enrolled and received ≥ 1 dose of T-DXd at the RDE. Patients were extensively pretreated (median, 7.5 prior therapies). The confirmed objective response rate by independent central review was 20/54 (37.0%; 95% CI, 24.3% to 51.3%) with median duration of response of 10.4 months (95% CI, 8.8 month to not evaluable). Most patients (53/54; 98.1%) experienced ≥ 1 treatment-emergent adverse event (TEAE; grade ≥ 3; 34/54; 63.0%). Common (≥ 5%) grade ≥ 3 TEAEs included decreases in neutrophil, platelet, and WBC counts; anemia; hypokalemia; AST increase; decreased appetite; and diarrhea. Three patients treated at 6.4 mg/kg suffered fatal events associated with T-DXd-induced interstitial lung disease (ILD)/pneumonitis as determined by an independent adjudication committee.
Conclusion: The novel HER2-targeted ADC, T-DXd, demonstrated promising preliminary antitumor activity in patients with HER2-low breast cancer. Most toxicities were GI or hematologic in nature. ILD is an important identified risk and should be monitored closely and proactively managed.
Figures
Comment in
-
Trastuzumab Deruxtecan Is Effective in HER2-Low Breast Cancer.Cancer Discov. 2020 Apr;10(4):488. doi: 10.1158/2159-8290.CD-RW2020-030. Epub 2020 Feb 28. Cancer Discov. 2020. PMID: 32111601
-
HER2-Targeted Therapies in HER2-Low-Expressing Breast Cancer.J Clin Oncol. 2020 Oct 1;38(28):3350-3351. doi: 10.1200/JCO.20.00657. Epub 2020 Jul 13. J Clin Oncol. 2020. PMID: 32658628 No abstract available.
-
Reply to T.J.A. Dekker.J Clin Oncol. 2020 Oct 1;38(28):3351-3352. doi: 10.1200/JCO.20.01212. Epub 2020 Jul 13. J Clin Oncol. 2020. PMID: 32658630 No abstract available.
References
-
- Koleva-Kolarova RG, Oktora MP, Robijn AL, et al. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat Rev. 2017;55:16–25. - PubMed
-
- Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. - PubMed
-
- Giuliani S, Ciniselli CM, Leonardi E, et al. In a cohort of breast cancer screened patients the proportion of HER2 positive cases is lower than that earlier reported and pathological characteristics differ between HER2 3+ and HER2 2+/HER2 amplified cases. Virchows Arch. 2016;469:45–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

