Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001
- PMID: 32058845
- PMCID: PMC7106984
- DOI: 10.1200/JCO.19.02767
Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001
Abstract
Purpose: Radiation dose to the neuroregenerative zone of the hippocampus has been found to be associated with cognitive toxicity. Hippocampal avoidance (HA) using intensity-modulated radiotherapy during whole-brain radiotherapy (WBRT) is hypothesized to preserve cognition.
Methods: This phase III trial enrolled adult patients with brain metastases to HA-WBRT plus memantine or WBRT plus memantine. The primary end point was time to cognitive function failure, defined as decline using the reliable change index on at least one of the cognitive tests. Secondary end points included overall survival (OS), intracranial progression-free survival (PFS), toxicity, and patient-reported symptom burden.
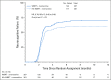
Results: Between July 2015 and March 2018, 518 patients were randomly assigned. Median follow-up for alive patients was 7.9 months. Risk of cognitive failure was significantly lower after HA-WBRT plus memantine versus WBRT plus memantine (adjusted hazard ratio, 0.74; 95% CI, 0.58 to 0.95; P = .02). This difference was attributable to less deterioration in executive function at 4 months (23.3% v 40.4%; P = .01) and learning and memory at 6 months (11.5% v 24.7% [P = .049] and 16.4% v 33.3% [P = .02], respectively). Treatment arms did not differ significantly in OS, intracranial PFS, or toxicity. At 6 months, using all data, patients who received HA-WBRT plus memantine reported less fatigue (P = .04), less difficulty with remembering things (P = .01), and less difficulty with speaking (P = .049) and using imputed data, less interference of neurologic symptoms in daily activities (P = .008) and fewer cognitive symptoms (P = .01).
Conclusion: HA-WBRT plus memantine better preserves cognitive function and patient-reported symptoms, with no difference in intracranial PFS and OS, and should be considered a standard of care for patients with good performance status who plan to receive WBRT for brain metastases with no metastases in the HA region.
Trial registration: ClinicalTrials.gov NCT02360215.
Figures


Comment in
-
Neurocognitive Outcomes for Patients With Brain Metastasis in the Modern Era: Benefit of Treatment With Hippocampal Avoidance Whole-Brain Radiotherapy Plus Memantine.J Clin Oncol. 2020 Apr 1;38(10):1003-1005. doi: 10.1200/JCO.19.03359. Epub 2020 Feb 14. J Clin Oncol. 2020. PMID: 32058847 No abstract available.
-
[Role of hippocampal avoidance during therapeutic whole-brain radiotherapy].Strahlenther Onkol. 2020 Sep;196(9):844-846. doi: 10.1007/s00066-020-01642-8. Strahlenther Onkol. 2020. PMID: 32519024 German. No abstract available.
-
Hippocampal Avoidance Whole-Brain Radiotherapy (WBRT) Versus WBRT in Patients With Brain Metastases: Were Hippocampi the Only Difference?J Clin Oncol. 2020 Oct 10;38(29):3453-3454. doi: 10.1200/JCO.20.00548. Epub 2020 Aug 12. J Clin Oncol. 2020. PMID: 32783669 No abstract available.
-
Hippocampal Avoidance and Memantine for Whole-Brain Radiotherapy: Long-Term Follow-Up Warranted.J Clin Oncol. 2020 Oct 10;38(29):3454-3455. doi: 10.1200/JCO.20.00747. Epub 2020 Aug 12. J Clin Oncol. 2020. PMID: 32783670 No abstract available.
-
Meeting the Burden of Proof to Justify Intensity-Modulated Radiation for Brain Metastases.J Clin Oncol. 2020 Oct 10;38(29):3452. doi: 10.1200/JCO.20.00486. Epub 2020 Aug 12. J Clin Oncol. 2020. PMID: 32783671 No abstract available.
-
Reply to S.G. Chun et al, A. Levy et al, and N. Andratschke et al.J Clin Oncol. 2020 Oct 10;38(29):3455-3457. doi: 10.1200/JCO.20.01167. Epub 2020 Aug 12. J Clin Oncol. 2020. PMID: 32783673 No abstract available.
-
Reducing Radiation-Induced Cognitive Toxicity: Sparing the Hippocampus and Beyond.Int J Radiat Oncol Biol Phys. 2021 Apr 1;109(5):1131-1136. doi: 10.1016/j.ijrobp.2021.01.001. Int J Radiat Oncol Biol Phys. 2021. PMID: 33714520 No abstract available.
References
-
- Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. - PubMed
-
- Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: Secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. 2015;1:457–464. - PubMed
-
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

