Mboat7 down-regulation by hyper-insulinemia induces fat accumulation in hepatocytes
- PMID: 32058943
- PMCID: PMC7026742
- DOI: 10.1016/j.ebiom.2020.102658
Mboat7 down-regulation by hyper-insulinemia induces fat accumulation in hepatocytes
Abstract
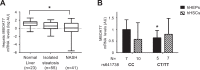
Background: Naturally occurring variation in Membrane-bound O-acyltransferase domain-containing 7 (MBOAT7), encoding for an enzyme involved in phosphatidylinositol acyl-chain remodelling, has been associated with fatty liver and hepatic disorders. Here, we examined the relationship between hepatic Mboat7 down-regulation and fat accumulation.
Methods: Hepatic MBOAT7 expression was surveyed in 119 obese individuals and in experimental models. MBOAT7 was acutely silenced by antisense oligonucleotides in C57Bl/6 mice, and by CRISPR/Cas9 in HepG2 hepatocytes.
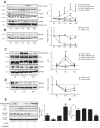
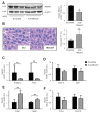
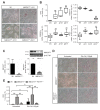
Findings: In obese individuals, hepatic MBOAT7 mRNA decreased from normal liver to steatohepatitis, independently of diabetes, inflammation and MBOAT7 genotype. Hepatic MBOAT7 levels were reduced in murine models of fatty liver, and by hyper-insulinemia. In wild-type mice, Mboat7 was down-regulated by refeeding and insulin, concomitantly with insulin signalling activation. Acute hepatic Mboat7 silencing promoted hepatic steatosis in vivo and enhanced expression of fatty acid transporter Fatp1. MBOAT7 deletion in hepatocytes reduced the incorporation of arachidonic acid into phosphatidylinositol, consistently with decreased enzymatic activity, determining the accumulation of saturated triglycerides, enhanced lipogenesis and FATP1 expression, while FATP1 deletion rescued the phenotype.
Interpretation: MBOAT7 down-regulation by hyper-insulinemia contributes to hepatic fat accumulation, impairing phosphatidylinositol remodelling and up-regulating FATP1.
Funding: LV was supported by MyFirst Grant AIRC n.16888, Ricerca Finalizzata Ministero della Salute RF-2016-02,364,358, Ricerca corrente Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico; LV and AG received funding from the European Union Programme Horizon 2020 (No. 777,377) for the project LITMUS-"Liver Investigation: Testing Marker Utility in Steatohepatitis". MM was supported by Fondazione Italiana per lo Studio del Fegato (AISF) 'Mario Coppo' fellowship.
Keywords: LPIAT1; NAFLD; Nash; Nonalcoholic fatty liver disease; Phosphatidylinositol; Phospholipid; Steatohepatitis.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. - PubMed
-
- Marchesini G., Brizi M., Morselli-Labate A.M., Bianchi G., Bugianesi E., McCullough A.J. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450–455. - PubMed
-
- Marra F., Gastaldelli A., Svegliati Baroni G., Tell G., Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14(2):72–81. - PubMed
-
- Dongiovanni P., Valenti L. Genetics of nonalcoholic fatty liver disease. Metab Clin Exp. 2016;65(8):1026–1037. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

