Principles of Cell Circuits for Tissue Repair and Fibrosis
- PMID: 32058955
- PMCID: PMC7005469
- DOI: 10.1016/j.isci.2020.100841
Principles of Cell Circuits for Tissue Repair and Fibrosis
Abstract
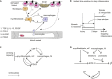
Tissue repair is a protective response after injury, but repetitive or prolonged injury can lead to fibrosis, a pathological state of excessive scarring. To pinpoint the dynamic mechanisms underlying fibrosis, it is important to understand the principles of the cell circuits that carry out tissue repair. In this study, we establish a cell-circuit framework for the myofibroblast-macrophage circuit in wound healing, including the accumulation of scar-forming extracellular matrix. We find that fibrosis results from multistability between three outcomes, which we term "hot fibrosis" characterized by many macrophages, "cold fibrosis" lacking macrophages, and normal wound healing. This framework clarifies several unexplained phenomena including the paradoxical effect of macrophage depletion, the limited time-window in which removing inflammation leads to healing, and why scar maturation takes months. We define key parameters that control the transition from healing to fibrosis, which may serve as potential targets for therapeutic reduction of fibrosis.
Keywords: In Silico Biology; Systems Biology; Tissue Engineering.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interest The authors declare no competing interests.
Figures






References
-
- Abergel R.P., Pizzurro D., Meeker C.A., Lask G., Matsuoka L.Y., Minor R.R., Chu M.-L., Uitto J. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J. Invest. Dermatol. 1985;84:384–390. - PubMed
-
- Adler M., Kohanim Y.K., Tendler A., Mayo A., Alon U. Continuum of gene-expression profiles provides spatial division of labor within a differentiated cell type. Cell Syst. 2019;8:43–52. - PubMed
-
- Alon, U., 2019. In: An Introduction to Systems Biology: Design Principles of Biological Circuits. CRC Press.
Grants and funding
LinkOut - more resources
Full Text Sources

