B cells infected with Type 2 Epstein-Barr virus (EBV) have increased NFATc1/NFATc2 activity and enhanced lytic gene expression in comparison to Type 1 EBV infection
- PMID: 32059024
- PMCID: PMC7046292
- DOI: 10.1371/journal.ppat.1008365
B cells infected with Type 2 Epstein-Barr virus (EBV) have increased NFATc1/NFATc2 activity and enhanced lytic gene expression in comparison to Type 1 EBV infection
Abstract
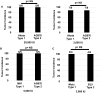
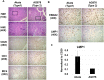
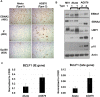
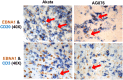
Humans are infected with two distinct strains (Type 1 (T1) and Type 2 (T2)) of Epstein-Barr virus (EBV) that differ substantially in their EBNA2 and EBNA 3A/B/C latency genes and the ability to transform B cells in vitro. While most T1 EBV strains contain the "prototype" form of the BZLF1 immediate-early promoter ("Zp-P"), all T2 strains contain the "Zp-V3" variant, which contains an NFAT binding motif and is activated much more strongly by B-cell receptor signalling. Whether B cells infected with T2 EBV are more lytic than cells infected with T1 EBV is unknown. Here we show that B cells infected with T2 EBV strains (AG876 and BL5) have much more lytic protein expression compared to B cells infected with T1 EBV strains (M81, Akata, and Mutu) in both a cord blood-humanized (CBH) mouse model and EBV-transformed lymphoblastoid cell lines (LCLs). Although T2 LCLs grow more slowly than T1 LCLs, both EBV types induce B-cell lymphomas in CBH mice. T1 EBV strains (M81 and Akata) containing Zp-V3 are less lytic than T2 EBV strains, suggesting that Zp-V3 is not sufficient to confer a lytic phenotype. Instead, we find that T2 LCLs express much higher levels of activated NFATc1 and NFATc2, and that cyclosporine (an NFAT inhibitor) and knockdown of NFATc2 attenuate constitutive lytic infection in T2 LCLs. Both NFATc1 and NFATc2 induce lytic EBV gene expression when combined with activated CAMKIV (which is activated by calcium signaling and activates MEF2D) in Burkitt Akata cells. Together, these results suggest that B cells infected with T2 EBV are more lytic due to increased activity of the cellular NFATc1/c2 transcription factors in addition to the universal presence of the Zp-V3 form of BZLF1 promoter.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




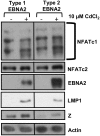
References
-
- Kieff E, Cohen JI, Longnecker R. Epstein-Barr Virus/Replication and Epstein-Barr Virus 6th ed In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1898–1959.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

