Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases
- PMID: 32059381
- PMCID: PMC7072842
- DOI: 10.3390/ijms21041219
Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases
Abstract
Adiponectin is the richest adipokine in human plasma, and it is mainly secreted from white adipose tissue. Adiponectin circulates in blood as high-molecular, middle-molecular, and low-molecular weight isoforms. Numerous studies have demonstrated its insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects. Additionally, decreased serum levels of adiponectin is associated with chronic inflammation of metabolic disorders including Type 2 diabetes, obesity, and atherosclerosis. However, recent studies showed that adiponectin could have pro-inflammatory roles in patients with autoimmune diseases. In particular, its high serum level was positively associated with inflammation severity and pathological progression in rheumatoid arthritis, chronic kidney disease, and inflammatory bowel disease. Thus, adiponectin seems to have both pro-inflammatory and anti-inflammatory effects. This indirectly indicates that adiponectin has different physiological roles according to an isoform and effector tissue. Knowledge on the specific functions of isoforms would help develop potential anti-inflammatory therapeutics to target specific adiponectin isoforms against metabolic disorders and autoimmune diseases. This review summarizes the current roles of adiponectin in metabolic disorders and autoimmune diseases.
Keywords: adiponectin; adiponectin isoform; anti-inflammatory; chronic kidney disease (CKD); inflammatory bowel disease (IBD); pro-inflammatory; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

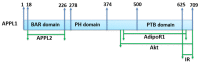
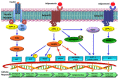
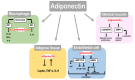

References
-
- Obata Y., Yamada Y., Takahi Y., Baden M.Y., Saisho K., Tamba S., Yamamoto K., Umeda M., Furubayashi A., Matsuzawa Y. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin. Endocrinol. (Oxf.) 2013;79:204–210. doi: 10.1111/cen.12041. - DOI - PubMed
-
- Berendoncks A.M.V., Stensvold D., Garnier A., Fortin D., Sente T., Vrints C.J., Arild S.S., Ventura-Clapier R., Wisløff U., Conraads V.M. Disturbed adiponectin—AMPK system in skeletal muscle of patients with metabolic syndrome. Eur. J. Prev. Cardiol. 2015;22:203–205. doi: 10.1177/2047487313508034. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

