Salicylic Acid Protects Photosystem II by Alleviating Photoinhibition in Arabidopsis thaliana under High Light
- PMID: 32059402
- PMCID: PMC7072977
- DOI: 10.3390/ijms21041229
Salicylic Acid Protects Photosystem II by Alleviating Photoinhibition in Arabidopsis thaliana under High Light
Abstract
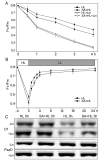
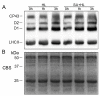
Salicylic acid (SA) is considered to play an important role in plant responses to environmental stresses. However, the detailed protective mechanisms in photosynthesis are still unclear. We therefore explored the protective roles of SA in photosystem II (PSII) in Arabidopsis thaliana under high light. The results demonstrated that 3 h of high light exposure resulted in a decline in photochemical efficiency and the dissipation of excess excitation energy. However, SA application significantly improved the photosynthetic capacity and the dissipation of excitation energy under high light. Western blot analysis revealed that SA application alleviated the decrease in the levels of D1 and D2 protein and increased the amount of Lhcb5 and PsbS protein under high light. Results from photoinhibition highlighted that SA application could accelerate the repair of D1 protein. Furthermore, the phosphorylated levels of D1 and D2 proteins were significantly increased under high light in the presence of SA. In addition, we found that SA application significantly alleviated the disassembly of PSII-LHCII super complexes and LHCII under high light for 3 h. Overall, our findings demonstrated that SA may efficiently alleviate photoinhibition and improve photoprotection by dissipating excess excitation energy, enhancing the phosphorylation of PSII reaction center proteins, and preventing the disassembly of PSII super complexes.
Keywords: Arabidopsis thaliana; chlorophyll fluorescence; photosystem; salicylic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

