Synthetic Approaches to A Challenging and Unusual Structure-An Amino-Pyrrolidine Guanine Core
- PMID: 32059504
- PMCID: PMC7070370
- DOI: 10.3390/molecules25040797
Synthetic Approaches to A Challenging and Unusual Structure-An Amino-Pyrrolidine Guanine Core
Abstract
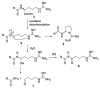
The synthesis of an unreported 2-aminopyrrolidine-1-carboxamidine unit is here described for the first time. This unusual and promising structure was attained through the oxidative decarboxylation of amino acids using the pair of reagents, silver(I)/peroxydisulfate (Ag(I)/S2O82-) followed by intermolecular (in the case of l-proline derivative) and intramolecular trapping (in the case of acyl l-arginine) by N-nucleophiles. The l-proline approach has a broader scope for the synthesis of 2-aminopyrrolidine-1-carboxamidine derivatives, whereas the intramolecular cyclization afforded by the l-acylarginines, when applied, results in higher yields. The former allowed the first synthesis of cernumidine, a natural alkaloid isolated in 2011 from Solanum cernuum Vell, as its racemic form.
Keywords: Cernumidine; amino acid decarboxylation; cyclic-guanidine; radical decarboxylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Toward the Synthesis of Strychnos Alkaloids: Effective Construction of Fused Cyclohexane and Pyrrolidine Portion of the Strychnos Skeleton via Domino Intermolecular and Intramolecular SN2 Cyclization.Chem Pharm Bull (Tokyo). 2025;73(1):46-57. doi: 10.1248/cpb.c24-00783. Chem Pharm Bull (Tokyo). 2025. PMID: 39864847
-
Synthesis of (+)-Ipalbidine Based on 6-exo-trig Radical Cyclization of a β-Amino Radical.J Org Chem. 2015 Oct 16;80(20):10294-8. doi: 10.1021/acs.joc.5b01890. Epub 2015 Oct 7. J Org Chem. 2015. PMID: 26402510
-
Stereodivergent access to cis- and trans-3,5-disubstituted 1,4-thiazane 1-oxides by cyclization of homochiral β-amino sulfoxides and sulfones. The preparation of isomeric ant venom alkaloids.Org Lett. 2013 Sep 6;15(17):4434-7. doi: 10.1021/ol4023003. Epub 2013 Aug 27. Org Lett. 2013. PMID: 23981062
-
[Syntheses of biologically active natural products using metal-mediated reactions as key reactions].Yakugaku Zasshi. 2000 Oct;120(10):1035-50. doi: 10.1248/yakushi1947.120.10_1035. Yakugaku Zasshi. 2000. PMID: 11082714 Review. Japanese.
-
Recent synthetic strategies for N-arylation of pyrrolidines: a potential template for biologically active molecules.Mol Divers. 2025 Apr;29(2):1851-1893. doi: 10.1007/s11030-024-10924-7. Epub 2024 Jul 24. Mol Divers. 2025. PMID: 39048884 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

