ROS in cancer therapy: the bright side of the moon
- PMID: 32060354
- PMCID: PMC7062874
- DOI: 10.1038/s12276-020-0384-2
ROS in cancer therapy: the bright side of the moon
Abstract
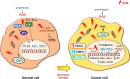
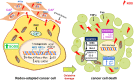
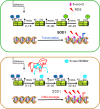
Reactive oxygen species (ROS) constitute a group of highly reactive molecules that have evolved as regulators of important signaling pathways. It is now well accepted that moderate levels of ROS are required for several cellular functions, including gene expression. The production of ROS is elevated in tumor cells as a consequence of increased metabolic rate, gene mutation and relative hypoxia, and excess ROS are quenched by increased antioxidant enzymatic and nonenzymatic pathways in the same cells. Moderate increases of ROS contribute to several pathologic conditions, among which are tumor promotion and progression, as they are involved in different signaling pathways and induce DNA mutation. However, ROS are also able to trigger programmed cell death (PCD). Our review will emphasize the molecular mechanisms useful for the development of therapeutic strategies that are based on modulating ROS levels to treat cancer. Specifically, we will report on the growing data that highlight the role of ROS generated by different metabolic pathways as Trojan horses to eliminate cancer cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. - PubMed
-
- Paradies G, et al. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circulation Res. 2004;94:53–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

