Novel Ocellatin Peptides Mitigate LPS-induced ROS Formation and NF-kB Activation in Microglia and Hippocampal Neurons
- PMID: 32060388
- PMCID: PMC7021831
- DOI: 10.1038/s41598-020-59665-1
Novel Ocellatin Peptides Mitigate LPS-induced ROS Formation and NF-kB Activation in Microglia and Hippocampal Neurons
Abstract
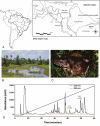
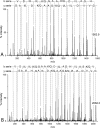
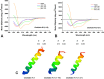
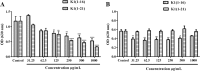
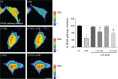
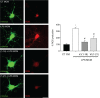
Cutaneous secretions of amphibians have bioactive compounds, such as peptides, with potential for biotechnological applications. Therefore, this study aimed to determine the primary structure and investigate peptides obtained from the cutaneous secretions of the amphibian, Leptodactylus vastus, as a source of bioactive molecules. The peptides obtained possessed the amino acid sequences, GVVDILKGAAKDLAGH and GVVDILKGAAKDLAGHLASKV, with monoisotopic masses of [M + H]± = 1563.8 Da and [M + H]± = 2062.4 Da, respectively. The molecules were characterized as peptides of the class of ocellatins and were named as Ocellatin-K1(1-16) and Ocellatin-K1(1-21). Functional analysis revealed that Ocellatin-K1(1-16) and Ocellatin-K1(1-21) showed weak antibacterial activity. However, treatment of mice with these ocellatins reduced the nitrite and malondialdehyde content. Moreover, superoxide dismutase enzymatic activity and glutathione concentration were increased in the hippocampus of mice. In addition, Ocellatin-K1(1-16) and Ocellatin-K1(1-21) were effective in impairing lipopolysaccharide (LPS)-induced reactive oxygen species (ROS) formation and NF-kB activation in living microglia. We incubated hippocampal neurons with microglial conditioned media treated with LPS and LPS in the presence of Ocellatin-K1(1-16) and Ocellatin-K1(1-21) and observed that both peptides reduced the oxidative stress in hippocampal neurons. Furthermore, these ocellatins demonstrated low cytotoxicity towards erythrocytes. These functional properties suggest possible to neuromodulatory therapeutic applications.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

