Dynamics of ACTH and Cortisol Secretion and Implications for Disease
- PMID: 32060528
- PMCID: PMC7240781
- DOI: 10.1210/endrev/bnaa002
Dynamics of ACTH and Cortisol Secretion and Implications for Disease
Abstract
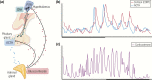
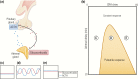
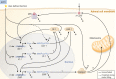
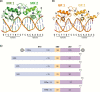
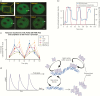
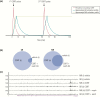
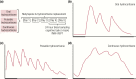
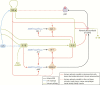
The past decade has seen several critical advances in our understanding of hypothalamic-pituitary-adrenal (HPA) axis regulation. Homeostatic physiological circuits need to integrate multiple internal and external stimuli and provide a dynamic output appropriate for the response parameters of their target tissues. The HPA axis is an example of such a homeostatic system. Recent studies have shown that circadian rhythmicity of the major output of this system-the adrenal glucocorticoid hormones corticosterone in rodent and predominately cortisol in man-comprises varying amplitude pulses that exist due to a subhypothalamic pulse generator. Oscillating endogenous glucocorticoid signals interact with regulatory systems within individual parts of the axis including the adrenal gland itself, where a regulatory network can further modify the pulsatile release of hormone. The HPA axis output is in the form of a dynamic oscillating glucocorticoid signal that needs to be decoded at the cellular level. If the pulsatile signal is abolished by the administration of a long-acting synthetic glucocorticoid, the resulting disruption in physiological regulation has the potential to negatively impact many glucocorticoid-dependent bodily systems. Even subtle alterations to the dynamics of the system, during chronic stress or certain disease states, can potentially result in changes in functional output of multiple cells and tissues throughout the body, altering metabolic processes, behavior, affective state, and cognitive function in susceptible individuals. The recent development of a novel chronotherapy, which can deliver both circadian and ultradian patterns, provides great promise for patients on glucocorticoid treatment.
Keywords: GR; HPA axis; MR; circadian; cortisol; dynamics; glucocorticoid; rhythms; ultradian.
© Endocrine Society 2020.
Figures

References
-
- Lightman SL, Conway-Campbell BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci. 2010;11(10):710–718. - PubMed
-
- Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. - PubMed
-
- Mohr E, Bahnsen U, Kiessling C, Richter D. Expression of the vasopressin and oxytocin genes in rats occurs in mutually exclusive sets of hypothalamic neurons. FEBS Lett. 1988;242(1):144–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

