Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications
- PMID: 32060542
- PMCID: PMC7341556
- DOI: 10.1210/endocr/bqaa017
Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications
Abstract
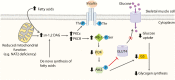
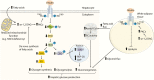
Insulin resistance (IR) is fundamental to the development of type 2 diabetes (T2D) and is present in most prediabetic (preDM) individuals. Insulin resistance has both heritable and environmental determinants centered on energy storage and metabolism. Recent insights from human genetic studies, coupled with comprehensive in vivo and ex vivo metabolic studies in humans and rodents, have highlighted the critical role of reduced mitochondrial function as a predisposing condition for ectopic lipid deposition and IR. These studies support the hypothesis that reduced mitochondrial function, particularly in insulin-responsive tissues such as skeletal muscle, white adipose tissue, and the liver, is inextricably linked to tissue and whole body IR through the effects on cellular energy balance. Here we discuss these findings as well as address potential mechanisms that serve as the nexus between mitochondrial malfunction and IR.
Keywords: insulin resistance; lipid accumulation; mitochondrial dysfunction; prediabetes; type 2 diabetes.
© Endocrine Society 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Cho NH, Shaw JE, Karuranga S, et al. . IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. - PubMed
-
- Cline GW, Petersen KF, Krssak M, et al. . Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341(4):240–246. - PubMed
-
- Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322(4):223–228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

