Japanese gastric cancer treatment guidelines 2018 (5th edition)
- PMID: 32060757
- PMCID: PMC7790804
- DOI: 10.1007/s10120-020-01042-y
Japanese gastric cancer treatment guidelines 2018 (5th edition)
Conflict of interest statement
Dr. Baba reports grants and personal fees from Chugai, grants and personal fees from Ono, grants and personal fees from Takeda, grants and personal fees from Eli Lilly, grants and personal fees from Taiho, grants from Medi Science Planning, grants and personal fees from MSD, grants from Astellas, grants and personal fees from Merck Serono, grants and personal fees from Daiichi Sankyo, grants and personal fees from Bristol-Myers, grants and personal fees from Esai, grants and personal fees from Bayer, grants and personal fees from Yakult, personal fees from Sumitomo Dainippon Pharma, personal fees from Kyowa Kirin, personal fees from Sanofi, personal fees from Tsumura, outside the submitted work. Dr. Kodera reports grants and personal fees from Taiho Pharma, grants and personal fees from Chugai Pharma, grants and personal fees from Takeda, grants and personal fees from MSD, grants from Nihon Kayaku, grants and personal fees from Yakult Pharma, grants and personal fees from Lilly Japan, grants and personal fees from Ono Pharma, grants from Kaken Pharma, grants and personal fees from Johnson & Johnson, grants and personal fees from Covidien, grants from EA Pharma, grants from Novartis, grants from KCI, grants from Maruho, grants from Daiichi Sankyo, grants and personal fees from Otsuka, grants from Tsumura, grants from Sawai, grants from Bristol, and grants from Sanofi outside the submitted work. Dr. Sano reports personal fees from Taiho Pharma, personal fees from Chugai Pharma, personal fees from Ono Pharma, personal fees from Lilly Japan, and personal fees from Yakult Pharma outside the submitted work.
Figures
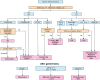
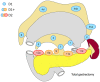
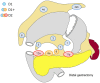
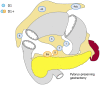



References
-
- Association Japanese Gastric Cancer. Japanese Classification of Gastric Carcinoma. 15. Tokyo: Kanehara Shuppan; 2017.
-
- Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 8. New Jersey: Wiley Blackwell; 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

