Bioinformatics design and experimental validation of influenza A virus multi-epitopes that induce neutralizing antibodies
- PMID: 32060794
- PMCID: PMC7222995
- DOI: 10.1007/s00705-020-04537-2
Bioinformatics design and experimental validation of influenza A virus multi-epitopes that induce neutralizing antibodies
Abstract
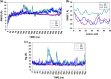
Pandemics caused by influenza A virus (IAV) are responsible for the deaths of millions of humans around the world. One of these pandemics occurred in Mexico in 2009. Despite the impact of IAV on human health, there is no effective vaccine. Gene mutations and translocation of genome segments of different IAV subtypes infecting a single host cell make the development of a universal vaccine difficult. The design of immunogenic peptides using bioinformatics tools could be an interesting strategy to increase the success of vaccines. In this work, we used the predicted amino acid sequences of the neuraminidase (NA) and hemagglutinin (HA) proteins of different IAV subtypes to perform multiple alignments, epitope predictions, molecular dynamics simulations, and experimental validation. Peptide selection was based on the following criteria: promiscuity, protein surface exposure, and the degree of conservation among different medically relevant IAV strains. These peptides were tested using immunological assays to test their ability to induce production of antibodies against IAV. We immunized rabbits and mice and measured the levels of IgG and IgA antibodies in serum samples and nasal washes. Rabbit antibodies against the peptides P11 and P14 (both of which are hybrids of NA and HA) recognized HA from both group 1 (H1, H2, and H5) and group 2 (H3 and H7) IAV and also recognized the purified NA protein from the viral stock (influenza A Puerto Rico/916/34). IgG antibodies from rabbits immunized with P11 and P14 were capable of recognizing viral particles and inhibited virus hemagglutination. Additionally, intranasal immunization of mice with P11 and P14 induced specific IgG and IgA antibodies in serum and nasal mucosa, respectively. Interestingly, the IgG antibodies were found to have neutralizing capability. In conclusion, the peptides designed through in silico studies were validated in experimental assays.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

