Oral Yohimbine as a New Probe Drug to Predict CYP2D6 Activity: Results of a Fixed-Sequence Phase I Trial
- PMID: 32060866
- PMCID: PMC7329762
- DOI: 10.1007/s40262-020-00862-6
Oral Yohimbine as a New Probe Drug to Predict CYP2D6 Activity: Results of a Fixed-Sequence Phase I Trial
Abstract
Objective: Yohimbine pharmacokinetics were determined after oral administration of a single oral dose of yohimbine 5 mg and a microdose of yohimbine 50 µg in relation to different cytochrome P450 (CYP) 2D6 genotypes. The CYP2D6 inhibitor paroxetine was used to investigate the influence on yohimbine pharmacokinetics. Microdosed midazolam was applied to evaluate a possible impact of yohimbine on CYP3A activity and the possibility of combining microdosed yohimbine and midazolam to simultaneously determine CYP2D6 and CYP3A activity.
Methods: In a fixed-sequence clinical trial, 16 healthy volunteers with a known CYP2D6 genotype [extensive (10), intermediate (2) and poor (4) metaboliser] received an oral dose of yohimbine 50 µg, yohimbine 5 mg at baseline and during paroxetine as a CYP2D6 inhibitor. Midazolam (30 µg) was co-administered to determine CYP3A activity at each occasion. Plasma concentrations of yohimbine, its main metabolite 11-OH-yohimbine, midazolam and paroxetine were quantified using validated liquid chromatography-tandem mass spectrometry assays.
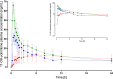
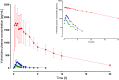
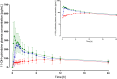
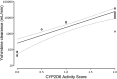
Results: Pharmacokinetics of yohimbine were highly variable and a CYP2D6 genotype dependent clearance was observed. After yohimbine 5 mg, the clearance ranged from 25.3 to 15,864 mL/min and after yohimbine 50 µg, the clearance ranged from 39.6 to 38,822 mL/min. A more than fivefold reduction in clearance was caused by paroxetine in CYP2D6 extensive metabolisers, while the clearance in poor metabolisers was not affected. Yohimbine did not alter CYP3A activity as measured by microdosed midazolam.
Conclusions: The pharmacokinetics of yohimbine were highly correlated with CYP2D6, which was further supported by the clearance inhibition caused by the CYP2D6 inhibitor paroxetine. With these data, yohimbine is proposed to be a suitable probe drug to predict CYP2D6 activity. In addition, the microdose can be used in combination with microdosed midazolam to simultaneously evaluate CYP2D6 and CYP3A activity without any interaction between the probe drugs and because the microdoses exert no pharmacological effects.
Clinical trial registration: EudraCT2017-001801-34.
Conflict of interest statement
Manuela Vay, Marleen Julia Meyer, Antje Blank, Gisela Skopp, Peter Rose, Mladen Vassilev Tzvetkov and Gerd Mikus have no conflicts of interest that are directly relevant to the content of this article.
Figures



References
-
- Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116(3):496–526. - PubMed
-
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–141. - PubMed
-
- McGraw J, Gerhardt A, Morris TC. Opportunities and obstacles in genotypic prediction of cytochrome P450 phenotypes. Expert Opin Drug Metab Toxicol. 2018;14(7):659–661. - PubMed
-
- Shah RR, Gaedigk A, Llerena A, Eichelbaum M, Stingl J, Smith RL. CYP450 genotype and pharmacogenetic association studies: a critical appraisal. Pharmacogenomics. 2016;17(3):259–275. - PubMed
-
- Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab. 2014;15(2):218–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

