Brain Penetration of Lorlatinib: Cumulative Incidences of CNS and Non-CNS Progression with Lorlatinib in Patients with Previously Treated ALK-Positive Non-Small-Cell Lung Cancer
- PMID: 32060867
- PMCID: PMC7028836
- DOI: 10.1007/s11523-020-00702-4
Brain Penetration of Lorlatinib: Cumulative Incidences of CNS and Non-CNS Progression with Lorlatinib in Patients with Previously Treated ALK-Positive Non-Small-Cell Lung Cancer
Abstract
Background: Lorlatinib is a potent, third-generation ALK/ROS1 tyrosine kinase inhibitor (TKI) designed to penetrate the blood-brain barrier.
Objective: We report the cumulative incidence of central nervous system (CNS) and non-CNS progression with lorlatinib in patients with ALK-positive non-small-cell lung cancer (NSCLC) previously treated with ALK TKIs.
Patients and methods: In an ongoing phase II study (NCT01970865), 198 patients with ALK-positive NSCLC with ≥ 1 prior ALK TKI were enrolled into expansion cohorts (EXP) based on treatment history. Patients received lorlatinib 100 mg once daily. Patients were analyzed for progressive disease, categorized as CNS or non-CNS progression, by independent central review. Cumulative incidence probabilities were calculated adopting a competing risks approach.
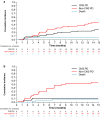
Results: Fifty-nine patients received crizotinib as their only prior ALK TKI (EXP2-3A); cumulative incidence rates (CIRs) of CNS and non-CNS progression were both 22% at 12 months in patients with baseline CNS metastases (n = 37), and CIR of non-CNS progression at 12 months was higher versus that for CNS progression in patients without baseline CNS metastases [43% vs. 9% (n = 22)]. In patients who received ≥ 1 prior second-generation ALK TKI [EXP3B-5 (n = 139)], CIR of non-CNS progression at 12 months was higher versus that for CNS progression in patients both with and without baseline CNS metastases (35% vs. 23% (n = 94) and 55% vs. 12% (n = 45), respectively).
Conclusions: Lorlatinib showed substantial intracranial activity in patients with pretreated ALK-positive NSCLC, with or without baseline CNS metastases, whose disease progressed on crizotinib or second-generation ALK TKIs. CLINICALTRIALS.
Gov identifier: NCT01970865.
Conflict of interest statement
TMB has received fees for consulting/advisory board roles for Guardant Health, Ignyta, Loxo, Moderna Therapeutics, and Pfizer Inc. ATS has received fees for consulting/advisory board roles from Ariad/Takeda, Bayer, Blueprint Medicines, Chugai, Daiichi Sankyo, EMD Serono, Genentech, Ignyta, KSQ Therapeutics, Loxo, Natera, Novartis, Pfizer, Roche, Taiho, and TP Therapeutics, and honoraria from Foundation Medicine, Guardant, Novartis, Pfizer, and Roche. AN has received fees for consult/advisory board roles from Boehringer Ingelheim, Roche, Pfizer, Bristol-Myers Squibb, Oryzon Genomics and travel expenses from Pfizer and Boehringer Ingelheim. MLJ has nothing to disclose. JFG has received fees for consulting/advisory board roles from Bristol-Myers Squibb, Genentech/Roche, Novartis, Loxo, Theravance, Clovis, Merck, Boehringer Ingelheim, and Incyte. HT, GP, YKP, and AA own stock in and are employees of Pfizer. EF has received fees for consulting/advisory board roles for Celgene, Eli Lilly, Guardant Health, and Takeda, and has served as a consultant and on the speakers’ bureau for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, and Roche.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

