GHR gene transcript heterogeneity may explain phenotypic variability in GHR pseudoexon (6Ψ) patients
- PMID: 32061156
- PMCID: PMC7077524
- DOI: 10.1530/EC-20-0026
GHR gene transcript heterogeneity may explain phenotypic variability in GHR pseudoexon (6Ψ) patients
Abstract
Objectives: The homozygous GH receptor (GHR) pseudoexon (6Ψ) mutation leads to growth hormone insensitivity (GHI) with clinical and biochemical heterogeneity. We investigated whether transcript heterogeneity (6Ψ-GHR to WT-GHR transcript ratio) and/or concurrent defects in other short stature (SS) genes contribute to this.
Methods: 6Ψ-GHR and WT-GHR mRNA transcripts of four 6Ψ patients (height SDS -4.2 to -3.1) and one control fibroblast were investigated by RT-PCR. Transcripts were quantified by qRT-PCR and delta delta CT analysis and compared using ANOVA with Bonferroni correction. In eleven 6Ψ patients, 40 genes known to cause GHI/SS were analysed by targeted next generation sequencing.
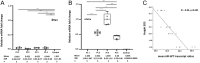
Results: RT-PCR confirmed 6Ψ-GHR transcript in the 6Ψ patients but not in the control. 6Ψ-GHR transcript levels were comparable in patients 1 and 3 but significantly different among all other patients. The mean 6Ψ:WT transcript ratios ranged from 29-71:1 for patients 1-4 and correlated negatively with height SDS (R = -0.85; P < 0.001). Eight deleterious variants in six genes were detected, but the number of gene hits did not correlate with the degree of SS in individual 6Ψ patients.
Conclusion: Variable amounts of 6Ψ- and WT-GHR transcripts were identified in 6Ψ patients but no 6Ψ transcript was present in the control. Higher 6Ψ:WT-GHR transcript ratio correlated with SS severity and may explain the phenotypic variability. Analysis of known SS genes suggested that phenotypic variation is independent of the genetic background. This is the first report of transcript heterogeneity producing a spectrum of clinical phenotypes in different individuals harbouring an identical homozygous genetic mutation.
Keywords: GHR pseudoexon; gene sequencing; growth hormone insensitivity; short stature; splicing.
Figures

References
-
- Domené HM, Bengolea SV, Martinez AS, Ropelato MG, Pennisi P, Scaglia P, Heinrich JJ, Jasper HG. Deficiency of the circulating insulin-like growth factor system associated with inactivation of the acid-labile subunit gene. New England Journal of Medicine 2004. 350 570–577. (10.1056/NEJMoa013100) - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

