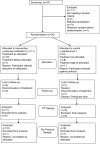
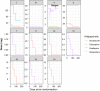
Reducing antipsychotic drugs in stable patients with chronic schizophrenia or schizoaffective disorder: a randomized controlled pilot trial
- PMID: 32062728
- PMCID: PMC7960583
- DOI: 10.1007/s00406-020-01109-y
Reducing antipsychotic drugs in stable patients with chronic schizophrenia or schizoaffective disorder: a randomized controlled pilot trial
Abstract
As the course of schizophrenic disorders is often chronic, treatment guidelines recommend continuous maintenance treatment to prevent relapses, but antipsychotic drugs can cause many side effects. It, therefore, seems reasonable to try to reduce doses in stable phases of the illness or even try to stop medication. We conducted a 26 weeks, randomized, rater blind, feasibility study to examine individualized antipsychotic dose reduction versus continuous maintenance treatment (Register Number: NCT02307396). We included chronic, adult patients with schizophrenia or schizoaffective disorder, who were treated with any antipsychotic drug except clozapine, who had not been hospitalized in the last 3 years and who were in symptomatic remission at baseline. The primary outcome was relapse of positive symptoms. Symptom severity, social functioning and side effects were also examined as secondary outcomes. 20 patients were randomized. Relapse rates in the two groups were not significantly different. No patient had to be hospitalized. One patient in the control group dropped out. The mean reduction of antipsychotic dose in the individualized dose-reduction group was 42%, however only one patient discontinued drug completely. There were no significant differences in efficacy or safety outcomes. This randomized trial provides evidence, that reduction of antipsychotic medication in chronic stable schizophrenic patients may be feasible. The results need to be confirmed in a larger trial with a longer follow-up period.
Keywords: Antipsychotic maintenance treatment; Chronic schizophrenia; Dose reduction; Randomized clinical trial; Relapse.
Conflict of interest statement
In the last 3 years MH has received speaker’s honoraria from Janssen. SL has received honoraria as a consultant/advisor and/or for lectures from LB Pharma, Otsuka, Lundbeck, Boehringer Ingelheim, LTS Lohmann, Janssen, Johnson&Johnson, TEVA, MSD, Sandoz, SanofiAventis, Angelini, Recordati, Sunovion, Geodon Richter. AH has received honoraria for consulting from Lundbeck, Janssen and Otsuka and for lecture from Janssen, Lundbeck and Otsuka. In the last 3 years, PR has received speaker’s honoraria from Janssen. SH has received speaker honoraria from Janssen-Cilag, Eli Lilly, Sanofi-Aventis, Otsuka, Lundbeck and Johnson & Johnson. SH has accepted travel or hospitality payment from Janssen-Cilag, Sanofi-Aventis, Johnson & Johnson, Pfizer, Bristol-Myers-Squibb, AstraZeneca, Lundbeck, Novartis and Eli Lilly. SH has participated in clinical trials sponsored or supported by Eli Lilly, Janssen Cilag, Johnson & Johnson, Bristol-Myers-Squibb, AstraZeneca, Lundbeck, Novartis, Servier, Pierre Fabre, Pfizer, Organon, Roche and Merck. SH has received honoraria for participation in advisory-boards or activities as a consultant from Lundbeck, Otsuka, Eli Lilly, Roche, Teva, Janssen and Johnson & Johnson. CL is SL spouse. For the remaining authors none were declared.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials