The splenium of the corpus callosum: embryology, anatomy, function and imaging with pathophysiological hypothesis
- PMID: 32062761
- PMCID: PMC7186255
- DOI: 10.1007/s00234-019-02357-z
The splenium of the corpus callosum: embryology, anatomy, function and imaging with pathophysiological hypothesis
Abstract
Background and purpose: The splenium of the corpus callosum is the most posterior part of the corpus callosum. Its embryological development, anatomy, vascularization, function, imaging of pathology, possible pathophysiological mechanisms by which pathology may develop and the clinical consequences are discussed.
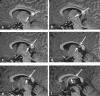
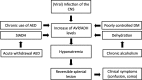
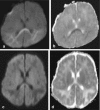
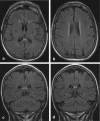
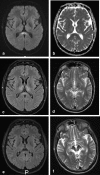
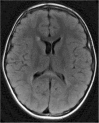
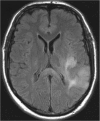
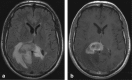
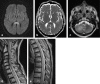
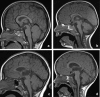
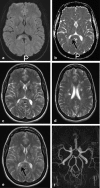
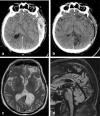
Methods: A literature-based description is provided on development, anatomy and function. MR and CT images are used to demonstrate pathology. The majority of pathology, known to affect the splenium, and the clinical effects are described in three subsections: (A) limited to the splenium, with elaboration on pathophysiology of reversible splenial lesions, (B) pathology in the cerebral white matter extending into or deriving from the splenium, with special emphasis on tumors, and (C) splenial involvement in generalized conditions affecting the entire brain, with a hypothesis for pathophysiological mechanisms for the different diseases.
Results: The development of the splenium is preceded by the formation of the hippocampal commissure. It is bordered by the falx and the tentorium and is perfused by the anterior and posterior circulation. It contains different caliber axonal fibers and the most compact area of callosal glial cells. These findings may explain the affinity of specific forms of pathology for this region. The fibers interconnect the temporal and occipital regions of both hemispheres reciprocally and are important in language, visuospatial information transfer and behavior. Acquired pathology may lead to changes in consciousness.
Conclusion: The development, location, fiber composition and vascularization of the splenium make it vulnerable to specific pathological processes. It appears to play an important role in consciousness.
Keywords: Anatomy; Consciousness; Corpus callosum; MRI; Pathophysiology; Splenium.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



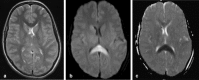
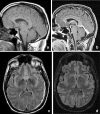
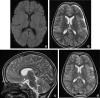
References
-
- Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 2010;52:447–477. - PubMed
-
- Takanashi J, Tada H, Kuroki H, Barkovich AJ. Delirious behavior in influenza is associated with a reversible splenial lesion. Brain Dev. 2009;31:423–426. - PubMed
-
- Barkovich AJ, Norman D. Anomalies of the corpus callosum: correlation with further anomalies of the brain. Am J Roentgenol. 1988;151:171–179. - PubMed
-
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. J Comp Neurol. 1968;132:45–72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

