Randomized Dose-Response Study of the New Dual Endothelin Receptor Antagonist Aprocitentan in Hypertension
- PMID: 32063059
- PMCID: PMC7098434
- DOI: 10.1161/HYPERTENSIONAHA.119.14504
Randomized Dose-Response Study of the New Dual Endothelin Receptor Antagonist Aprocitentan in Hypertension
Abstract
This study examined the dose-response characteristics of aprocitentan, a dual endothelin A/endothelin B receptor antagonist, in patients with essential hypertension. In a randomized, double-blind, parallel study design, eligible patients with a sitting diastolic blood pressure (BP) of 90-109 mm Hg received aprocitentan 5, 10, 25, or 50 mg, placebo, or lisinopril 20 mg as a positive control once daily for 8 weeks. Multiple automated office BP readings were obtained with patients resting unattended (unattended automated office BP) at baseline, weeks 2, 4, and 8. Ambulatory BP was monitored for 24 hours at baseline and week 8. After a single-blind placebo run-in period, 490 eligible patients were randomized to the double-blind phase, with 409 patients completing 8 weeks of therapy per protocol. Aprocitentan 10, 25, and 50 mg decreased sitting systolic/diastolic unattended automated office BP from baseline to week 8 (placebo-corrected decreases: 7.05/4.93, 9.90/6.99, and 7.58/4.95 mm Hg, respectively, P≤0.014 versus placebo), compared with an unattended automated office BP reduction of 4.84/3.81 mm Hg with lisinopril 20 mg. For patients with valid ambulatory BP, aprocitentan 10, 25, and 50 mg significantly decreased placebo-corrected 24-hour BP by 3.99/4.04, 4.83/5.89, and 3.67/4.45 mm Hg, respectively. Incidence of adverse events was similar in the aprocitentan groups (22.0%-40.2%) and the placebo group (36.6%). Aprocitentan produced dose-dependent decreases in hemoglobin, hematocrit, albumin, and uric acid, an increase in estimated plasma volume, but no change in weight versus placebo. These findings support further investigation of aprocitentan at doses of 10 to 25 mg in hypertension. Registration- URL: https://www.clinicaltrials.gov; Unique identifier: NCT02603809.
Keywords: aprocitentan; blood pressure; endothelin; essential hypertension.
Figures

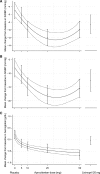
References
-
- Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. - PubMed
-
- FDA. Hypertension: Conducting studies of drugs to treat patients on a background of multiple antihypertensive drugs guidance for industry. 2018. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed July 16, 2019.
-
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. - PubMed
-
- Iglarz M, Clozel M. At the heart of tissue: endothelin system and end-organ damage. Clin Sci (Lond) 2010;119:453–463. doi: 10.1042/CS20100222. - PubMed
-
- Schiffrin EL. Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens. 2001;14(6)(pt 2):83S–89S. doi: 10.1016/s0895-7061(01)02074-x. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

