Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes
- PMID: 32063863
- PMCID: PMC7000657
- DOI: 10.3389/fphys.2019.01607
Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes
Abstract
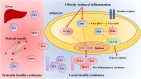
Obesity is one of the major health burdens of the 21st century as it contributes to the growing prevalence of its related comorbidities, including insulin resistance and type 2 diabetes. Growing evidence suggests a critical role for overnutrition in the development of low-grade inflammation. Specifically, chronic inflammation in adipose tissue is considered a crucial risk factor for the development of insulin resistance and type 2 diabetes in obese individuals. The triggers for adipose tissue inflammation are still poorly defined. However, obesity-induced adipose tissue expansion provides a plethora of intrinsic signals (e.g., adipocyte death, hypoxia, and mechanical stress) capable of initiating the inflammatory response. Immune dysregulation in adipose tissue of obese subjects results in a chronic low-grade inflammation characterized by increased infiltration and activation of innate and adaptive immune cells. Macrophages are the most abundant innate immune cells infiltrating and accumulating into adipose tissue of obese individuals; they constitute up to 40% of all adipose tissue cells in obesity. In obesity, adipose tissue macrophages are polarized into pro-inflammatory M1 macrophages and secrete many pro-inflammatory cytokines capable of impairing insulin signaling, therefore promoting the progression of insulin resistance. Besides macrophages, many other immune cells (e.g., dendritic cells, mast cells, neutrophils, B cells, and T cells) reside in adipose tissue during obesity, playing a key role in the development of adipose tissue inflammation and insulin resistance. The association of obesity, adipose tissue inflammation, and metabolic diseases makes inflammatory pathways an appealing target for the treatment of obesity-related metabolic complications. In this review, we summarize the molecular mechanisms responsible for the obesity-induced adipose tissue inflammation and progression toward obesity-associated comorbidities and highlight the current therapeutic strategies.
Keywords: adaptive immunity; adipose tissue inflammation; diabetes; inflammatory triggers; innate immune system; insulin resistance; low-grade inflammation; obesity.
Copyright © 2020 Zatterale, Longo, Naderi, Raciti, Desiderio, Miele and Beguinot.
Figures

References
-
- American Diabetes Association (2018). Classification and diagnosis of diabetes: standards of medical care in diabetes. Diabetes Care 41(Suppl. 1), S13–S27. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

